Revolutionizing regenerative medicine with engineered biomaterials
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
“The cornea is the windshield of the eye,” said Ramez Haddadin, assistant professor of ophthalmology at Northwestern University Feinberg School of Medicine in Chicago. Damage to the
structure and shape of the cornea is a common cause of blindness. At present, the only treatment to restore vision involves replacing the cornea, or part of it, with corneal tissue from a
donor. The demand for donor corneas vastly outstrips supply. It is estimated that there are over 12 million people waiting for a corneal transplant worldwide1. Moreover, the outcome of these
transplants is not always perfect, further emphasizing the need for more-effective treatment options. “Every donated cornea is different; it may not match the shape of the patient’s eye
leading to other complications, or it could be rejected by the patient’s immune system, leaving them back at square one,” Haddadin explained. INNOVATIVE OFF-THE-SHELF SOLUTION Pandorum
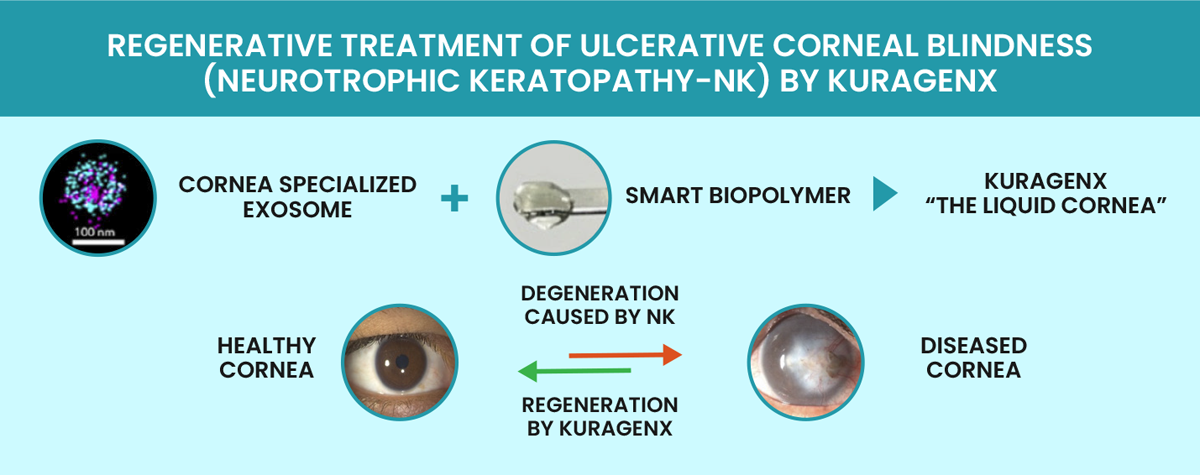
Technologies is developing a transformative solution to corneal transplants. The company’s flagship product, the liquid cornea Kuragenx, has been designed to stimulate the repair and
regeneration of damaged corneal tissue. Kuragenx is a transparent viscous liquid comprising cell-derived vesicles called exosomes with enhanced regenerative properties in a biopolymeric
solution that acts as a temporary scaffold supporting epithelial cell growth and sustained exosome release. Kuragenx is administered dropwise to the wound site after a surgeon has removed
the diseased tissue. Post-application, the liquid spreads, solidifies within a few minutes under visible light and integrates into the host cornea in a suture-less manner. Extensive work in
New Zealand rabbits with corneal wounds that mimic post-ulcerative corneal scarring in humans2 showed that Kuragenx could restore corneal health within a few months3. Kuragenx stimulated
re-epithelialization, re-innervation and stromal regeneration so the structure and physiology of wounded corneas resembled those of normal corneas. As a result of these promising findings,
Pandorum has been working with clinical collaborators in the US and India to pave the way for Kuragenx’s first-in-human trials. In India, ophthalmologist and stem cell biologist Virender
Sangwan leads the clinical study at Dr Shroff’s Charity Eye Hospital, New Delhi. Kuragenx has been designated as a combination product by the US Food and Drug Administration (FDA)’s Center
for Biologics Evaluation and Research and was recently awarded orphan drug designation for the treatment of advanced neurotrophic keratopathy (NK), a severe degenerative corneal disease
caused by impairment of corneal sensory innervation. “This treatment could help NK patients in ways that no other therapies can,” Haddadin said. The first-in-human, phase 1/2a study of
Kuragenx will take place at Northwestern University Feinberg School of Medicine and Dr Shroff’s Charity Eye Hospital from 2024. “We will start by treating a very small pool of patients with
very severe disease, but if Kuragenx works as expected, I anticipate many more people will be able to benefit from this therapy,” said Haddadin. PANDORUM’S TISSUE-AGNOSTIC APPROACH Pandorum
was co-founded in 2011 by Tuhin Bhowmick and Arun Chandru in Bangalore, India. The company and its US arm (Pandorum International Inc.) now include over 40 employees who are using its
proprietary technology platform to develop regenerative medicines for diseases associated with tissue inflammation and fibrosis. Pandorum’s technology platform can combine therapeutic
exosomes with biopolymers that mimic extracellular-matrix materials, representing a unique approach to restore and repair tissues such as the cornea, lung and liver. Exosomes are natural
mediators of cell-to-cell communication. The stability, bioavailability, circulation half-life and low immunogenicity of exosomes make them promising carriers for drug delivery4. By
optimizing the expression patterns of clinical-grade human stem cells, Pandorum can produce exosomes enriched with specific cargoes that stimulate tissue regeneration. “We can produce
exosome variants with anti-fibrotic, anti-inflammatory and neurogenic properties to suit the needs of specific tissues,” Bhowmick explained. In addition, Pandorum has developed a range of
biomaterials that can mimic the mechanical and chemical properties of the extracellular environment of target tissues and trigger tissue-specific cellular activity. “The properties of our
tissue-mimetic, biopolymer-containing hydrogels, such as transparency, biocompatibility and biodegradability, can be fine-tuned for various applications,” said Bhowmick. Through the
effective delivery of exosomes packed with therapeutic factors, Pandorum is building a pipeline of products to heal damaged tissues. “Our goal is to make regenerative medicine a mainstream
reality and improve the lives of millions of patients suffering from a wide range of injuries and diseases,” Bhowmick concluded.