Readers of histone modifications
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Histone modifications not only play important roles in regulating chromatin structure and nuclear processes but also can be passed to daughter cells as epigenetic marks.
Accumulating evidence suggests that the key function of histone modifications is to signal for recruitment or activity of downstream effectors. Here, we discuss the latest discovery of
histone-modification readers and how the modification language is interpreted. SIMILAR CONTENT BEING VIEWED BY OTHERS MULTIFUNCTIONAL HISTONE VARIANTS IN GENOME FUNCTION Article 13 August
2024 DECODING CHROMATIN STATES BY PROTEOMIC PROFILING OF NUCLEOSOME READERS Article Open access 06 March 2024 HISTONE POST-TRANSLATIONAL MODIFICATIONS — CAUSE AND CONSEQUENCE OF GENOME
FUNCTION Article 25 March 2022 INTRODUCTION Most eukaryotic DNA is wrapped around histone proteins to form chromatin, a stable structure that limits DNA accessibility to its binding
partners. Histones are subject to posttranslational modifications (PTMs), and these modifications are important parts of regulatory circuits that control chromatin dynamics and the
activities taking place with the underlying DNA 1. Histone modifications also function as epigenetic passengers that can be inherited by daughter cells to maintain lineage-specific
transcription profiles 2. Thus, understanding functions of histone modifications has become a central focus of the chromatin field 3. Modifications on histones can directly influence
chromatin structure. For instance, acetylation on lysine residues can reduce the positive charge of histones, thereby weakening their interaction with negatively charged DNA and increasing
nucleosome fluidity 4. Even acetylation on a single residue (H4K16) can alter the compaction level of a nucleosomal array 5. Moreover, the diverse chemical moieties involved in histone PTM
and modification sites have led to the proposal of the histone code hypothesis 6, 7. It postulates that PTMs function as a signal platform to recruit effector modules to local chromatin, and
it is the effectors/readers that ultimately determine the functional outcome of certain PTMs. Over the past 10 years, the field has discovered multiple families of conserved domains that
recognize modified histones. Biochemical and biophysical studies have revealed a wealth of details on how individual domains interact with modified histone peptides 8. However, deciphering
the language of histone PTM is much more than matching histone marks with their binding partners. The biological outcome of certain PTMs often heavily depends on the chromatin and cellular
context of such modifications 9. In this article, we will provide an overview of recent advances in reading histone modifications and highlight studies that influence the view of the field.
We first review key experimental approaches that led to identification of PTM readers. Although a comprehensive list of PTM-recognition domains is provided, we will mainly focus on common
themes of interactions and the importance of chromatin context. Next, we discuss how individual recognition modules are utilized by a functional complex to interpret PTM language and explain
why multivalent recognition emerges as a prevalent mechanism. Finally, we summarize how to regulate PTM reading and functional outcomes of histone modifications. LOOKING FOR READERS Bromo
domains were initially found in nuclear histone acetyltransferases (HATs) but not in cytoplasmic HATs, which led to the speculation that bromo domains may recognize acetylated histones in
chromatin. Dhalluin _et al_. went on to demonstrate for the first time that bromo domains preferentially interact with H4K8-acetylated histone peptides 10. Since then, samplings between
conserved domains in chromatin-related proteins and chemically modified histone peptides revealed several PTM readers, such as the double-bromo domain of TAF1 (which recognizes acetylated
histones) 11 and the chromo domain of HP1 (recognizing H3K9me) 12. This trend continued until a high-throughput candidate-based approach was developed. Protein microarrays that carry a large
number of chromatin-related domains were produced to accelerate the reader-screening process. Using a series of fluorescence-labeled modified histone peptide probes, Tudor and MBT domains
were identified as new classes of methyl-lysine (MeK) readers 13. As a complementary method, peptide microarrays that contain modified and unmodified histone peptides were used to discover
several new members of the Tudor domain family that can read MeK 14. Despite robustness of the approach described above, unbiased screening methods are more desirable to discover
unpredictable matches between PTMs and their readers. In one strategy, immobilized histone peptides were used as baits to retrieve their recognition proteins from nuclear extracts, and MDC1
was identified as a novel binder for phosphorylated H2AX (γH2AX) peptides 15. Conversely, a chromatin-associated protein, 53BP1, was used as a bait to look for specifically modified histones
from purified native core histones 16. The authors discovered that 53BP1 preferentially binds to H3K79me, based on mass-spectrometry analysis 16. Recently, a quantitative proteomics method
has been developed to improve unbiased PTM reader screens 17. In this so-called SILAC (Stable Isotope Labeling by Amino acids in Cell culture) technology, nuclear extracts from
“Heavy”-labeled cells are incubated with modified peptides, whereas “Light”-labeled extracts are incubated with unmodified peptides. Pull-down assays are performed separately. Enriched
proteins are mixed before being analyzed by mass spectrometry, which allows a quantitative comparison between binders for unmodified histones and modification-specific binders 17. Using this
method, the authors provided a comprehensive list of readers for several transcription-related modifications such as active marks H3K4me3 and H3K36me3 as well as repressive marks H3K9me3,
H3K27me3 and H4K20me3 17. Recently, this SILAC technology has been further developed to screen for PTM readers in the context of nucleosomes 18. In summary, chemically modified histone
peptides were used to identify almost all PTM readers so far. Weak interactions between PTM reading domains and short peptides likely reduce the dynamic range of such assays 8, thus
neglecting potential PTM binders that prefer a nucleosomal context. Future screens utilizing native complexes in combination with modified nucleosomes should provide additional avenues to
discover PTM readers. READING MODULES OF HISTONE MODICATIONS We will first discuss individual domains that recognize a unique PTM signal or, in a less stringent way, a bunch of similar
signals. Without considering the role of DNA and chromatin context, interactions between modified histone peptides and recognition domains are similar to generic protein-protein
interactions. Readers typically provide an accessible surface (such as a cavity or surface groove) to accommodate a modified histone residue, and determine the modification (acetylation vs
methylation) or state specificity (such as mono- vs trimethylation of lysine). Readers also interact with the flanking sequence of the modified amino acid in order to distinguish sequence
context. In this section, we will introduce the reading modules of individual PTM signals from a structural perspective. Although the folding of individual domains is critical for complex
assembly and other functions, it is beyond the scope of this review. For simplicity, we concentrate on the interface that recognizes modified histones, particularly for figure illustrations.
LYSINE ACETYLATION Although histone acetylation takes place at multiple lysines, genetic experiments suggest that many acetylation marks display redundant functions 19, 20. In addition,
most acetylation writers and erasers – HATs and histone deacetylases (HDACs) – modify several lysines, and multiple enzymes target common sites 21. Therefore, it was proposed that histone
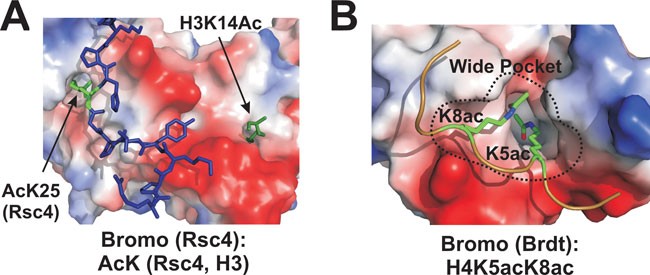
acetylation may function through cumulative effects 20. Acetylated lysines (AcKs) can be recognized by bromo domains 10 and the tandem PHD domain 22, 23. Many bromo domains use a narrow but
deep cavity to accommodate acetyl-lysine and its long side-chain 10, 24 (Figure 1A). The bromo domain 1 of Brdt has a much wider pocket, which holds two AcKs simultaneously (Figure 1B) 25;
whereas the tandem PHD has a shallow cage 23. All known AcK binding pockets are hydrophobic with hydrogen bond capacity at the bottom. AcK intercalates into the pocket mainly through a
hydrogen bond and the interaction is stabilized by a network of water-mediated intermolecular hydrogen bonds 23. Many bromo domains bind to multiple acetylated histones and the tandem PHD
domain of human DPF3b also prefers acetylated H3 and H4 22, indicating the lack of unique sequence recognition by these readers. This limited specificity is likely because the flanking
peptides of AcK tend to make less defined contacts with the surface of the readers (Figure 1A). Since the interaction between AcK and its readers is relatively weak, multiple domains working
in tandem are common. For instance, the chromatin-remodeling complex, RSC, has three bromo domain-containing subunits (Rsc1, Rsc2 and Rsc4) 26, the SAGA-HAT complex contains two-bromo
domain proteins (Gcn5 and Spt7) and the polybromo (PB) protein alone consists of six-bromo domains 27. Multiple copies of readers also favor cumulative effects of histone acetylation. LYSINE
METHYLATION Lysine methylation is one of the most stable histone marks, and it presents four types of signals: unmethylated (me0), mono- (me1), di- (me2) and tri- (me3) methylation. We
include unmethylated lysine here as a part of the MeK-signaling group because methylation is the only known PTM at those residues and almost all known me0 readers are sensitive to addition
of methyl group on the lysine. Thus, we consider them bona fide sensors for MeK. Domains that recognize histone MeK include PHD, chromo, WD40, Tudor, double/tandem Tudor, MBT, Ankyrin
Repeats, zf-CW and PWWP domains (Table 1), a long list that may continue to grow in coming years. Although different binders are folded differently to fulfill other structural requirements,
for the domains that recognize the same mark, their binding surfaces remarkably resemble each other (such as in Figure 2A and 2B). Unlike acetylation, methylation is highly site-specific and
is maintained by histone methyltransferases and demethylases that possess stronger site-specificity than HAT and HDAC 28. Due to the diversity of both methyl lysine signals and their
recognition modules, instead of breaking down each family of recognition domains, we will generalize some common themes shared by multiple MeK binders and highlight a few representative
examples. BINDING POCKETS Most MeK binders form an aromatic cage to accommodate MeK with its long hydrophobic side-chain. Primary functions of these pockets are to discriminate different
PTMs and methylation states. MeK-binding pockets are relatively static on histone binding – an exception being the WD40 domain of EED, which will be discussed later. Binders for mono- and
dimethylation tend to have a small keyhole-like cavity (Figure 2C) that limits the access of the larger trimethyl group. In contrast, binders for di- and trimethylation often use a wider and
more accessible surface groove as binding pockets (Figure 2A, 2B and 2F and Figure 3), which may also result in less stringency for the specific methylation states. Binding pockets for MeK
can be loosely defined either as half aromatic cages (Figure 2D II and III) or as full aromatic cages (Figure 2D IV and V), based on the number of aromatic residues within the pocket. All
residues within the pocket engage in binding with MeK, as a point mutation of any residue severely compromises the substrate-binding ability 8, 29. Tri- and dimethylated ammonium groups were
secured mostly through van der Waals and cation-π interactions. Although no evidence suggests that the number of aromatic residues correlates with the binding capacity of MeK and the
overall affinity to histone peptides, there is an example in which the tryptophan residue seems to generate a stronger cation-π interaction than the tyrosine in a mutant protein (W868Y) 30.
This result implies that the tryptophan at the orthogonal position of MeK (Figure 2D III) contributes to a stronger binding pocket than the corresponding tyrosines in other aromatic cages
(Figure 2D II, IV and V). Unmethylated lysine (UmK) readers do not have apparent pockets (Figure 2D I and 2E). On reader binding, UmK is stabilized by intermolecular hydrogen bonds (e.g., in
BHC80 PHD, the bonds between the epsilon amino group of H3K4 and D489 or E488 of PHD, Figure 2E). However, addition of a methyl group would clash with the binding surface 31. This spatial
restriction also specifies recognition of UmK by other K4me0 readers, such as the ADD domain of Dnmt3L 32 and the PHD domain of AIRE 33. The recognition of methyl states can be utilized in
two ways. At some lysines, different methyl-states recruit different sets of effectors. For instance, Pdp1 binds to H4K20me1 for cell-cycle regulation, whereas Crb2 recognizes H4K20me2 to
control a DNA damage checkpoint 34. However, at other sites, methyl states only control the binding strength of the same chromatin regulators. For example, Rpd3S binds to K36me1 nucleosomes
at a similar affinity to the unmodified ones, K36me2 shows stronger binding and K36me3 displays the highest affinity 35. FLANKING SEQUENCE INTERACTIONS Readers of MeK make multiple contacts
with flanking amino acids to determine the sequence context. Free histone peptides are normally unstructured in solution. However, on binding, they are induced into a β-sheet conformation,
which is aligned antiparallel to the surface groove of the readers (Figure 2E). This pairing interaction not only increases the overall affinity but also projects MeK in a specific
orientation to influence its pocket binding (Figure 2E and 2F). Flanking-sequence contacts can make MeK binders highly selective for the sequence context. For example, all three H3K4 readers
shown in Figure 2A, 2B and 2E adopt a similar surface where H3R2 and H3K4 are divided into two separate binding pockets. H3R2 contributes significantly to the binding of TAF3 PHD (Figure
2A) and the JMJD2A double-Tudor domain (Figure 2B) but not for BHC80 PHD (where H3R8 plays a similar role by interacting with D489; Figure 2E). In contrast, flanking-sequence interaction
enables the WD40 domain of EED to broadly recognize a group of similar histone modifications 36, 37. EED has two small hydrophobic pockets (labeled as P1 and P2 in Figure 3B), which only
accommodate small residues at the –2 or +2 position (Figure 3A) relative to MeK. This feature eliminates the binding of histone peptides that have bulky residues at these positions – such as
the three active histone marks shown in Figure 3A. In contrast, it favors the binding of repressive mark histone peptides in which either an alanine is present at the -2 position or a
leucine is at the +2 position (H4K20) 36, 37. Lastly, MeK binders that do not make extensive contacts with the flanking sequence display a promiscuous PTM recognition pattern – such as the
MBT domain of L3MBTL1, which binds to multiple lysines in the me1 or me2 states 38. HISTONE END EFFECTS MeK that locates near the end of a histone peptide is easy to read. This is because:
(1) the histone termini can be buried into a pocket, which greatly contributes to the overall affinity (Figure 2A, 2B and 2E) and (2) without interference from adjacent peptide extension,
the histone peptides can be fit into a variety of conformations, thus attracting more readers. A good example is the H3K4 methylation, which has strong and very well-studied reading modules.
In summary, it is the combination of the above three elements that determines the strength and specificity of a particular MeK reader. For instance, the PHD domain of TAF3 possesses a
superior aromatic cage in which a tryptophan is at the base (Figure 2D III), as discussed above, H3A1 is buried in a deeper pocket than in JMJD2A (Figure 2B) or BHC80 (Figure 2E) and lastly,
H3R2 interacts with a more negatively charged surface 30. These features collectively make TAF3 PHD one of the strongest binders among known PTM readers. ARGININE METHYLATION
Methylarginines (MeR) are found in three different forms: monomethylation (me1), symmetrical dimethylation (me2s) and asymmetrical dimethylation (me2a). Although arginine methylation has
been linked to transcription for years, the readers of this mark were only reported recently. The ADD domain (containing a PHD motif) of the DNA methyltransferase Dnmt3a recognizes H4R3me2s
but not H4R3me2a, thereby linking histone MeR to DNA methylation and gene repression 39. The Tudor domain of TDRD3 is a reader for H3R17me2a and H4R3me2a 40. TDRD3 acts as a transcription
coactivator and is enriched at transcription start sites 40, which link MeR to active transcription. Due to limited information about MeR readers, it is too early to evaluate their state
specificity and sequence fidelity in general. However, the ADD domain clearly discriminates the symmetry of MeR 39. In yeast, H3R2me1 and H3R2me2 display distinct localization patterns and
transcriptional outputs. H3R2me1 is linked to activation while H3R2me2 is involved with repression 41, suggesting that MeR readers are likely specific to methyl states. SERINE
PHOSPHORYLATION Protein domains that recognize phosphorylated amino acids in a non-histone context are well characterized and include SH2, BRCT, WW, FHA, WD40, 14-3-3 and LRR domains.
However, only two readers have been identified for phosphorylated serine (PhS) in histones. The BRCT domain of MDC1 binds to PhS near the C-terminus of histone H2AX 15. The PhS peptide docks
at the inter-bridge between two lobes of BRCT (Figure 4A). PhS is stabilized by several hydrogen bonds. The C-terminus of the peptide is anchored by a surface pocket to provide additional
affinity 15. Curiously, phosphorylation of the H2AX family typically takes place at a conserved SQ(E/D)X motif 42. However, the glutamine does not appear to be important for BRCT contact
(Figure 4A). PhS is also read by the 14-3-3 family. Mammalian 14-3-3ζ recognizes H3S10ph peptide using a deep scaffold (Figure 4B) 43. PhS is secured through multiple hydrogen bonds and
H3K9ac does not disrupt peptide contacts due to a large binding surface (Figure 4B). Thus, it was concluded that H3K9ac does not influence the binding of 14-3-3 to PhS 43. Interestingly, the
binding of the yeast 14-3-3 proteins Bmh1 and Bmh2 to H3S10ph peptides is stimulated by H3K14ac, and H3K14ac is important for the recruitment of Bmh1 _in vivo_ 44. Future structural
analysis would provide more insight into how the same family of readers responds differently to the PTM near its primary target. LYSINE UBIQUITINATION No histone ubiquitination reading
module has been clearly identified yet. Unlike other PTMs, the ubiquitin (Ub) moiety is relatively large in size. Typical Ub binders recognize either the surface of Ub (such as the
hydrophobic patch) or the C-terminal region where Ub is conjugated to target proteins 45. Thus, finding specific readers for ubiquitinated histones has proven to be difficult. A recent
discovery that histones can be mono-ubiquinated and poly-ubiquitinated 46 has further complicated the search for readers. However, one potential candidate has emerged from a study showing
that incorporation of the Cps35 subunit into a histone methyltransferase complex, COMPASS, depends on ubiquitination of H2B 47. This result implied that Cps35 may be the direct reader of
H2BUb or may associate with a specific reader, a notion confirmed by another study using a H2B ubiquitination-defective mutant 48. CHROMATIN CONTEXT Under physiological conditions where
modified histones are embedded in chromatin, PTM signals might be presented differently than in free peptides. For example, once wrapped in a nucleosome, K79me2 appears to be less accessible
(Figure 5) 49. Given this structural constraint, the flanking sequence of K79me2 could not be freely changed into a conformation that favors the binding of readers, and neighboring residues
of H3K79 are not fully exposed. Therefore, it is important to examine PTM recognition by reading modules in a more relevant chromatin context in the future. READING MODES
Chromatin-associated complexes typically contain multiple PTM readers to respond to different signals. Here, we will discuss how each individual reading module coordinately contributes to
the targeting of a complex to modified chromatin. MONOVALENT RECOGNITION – “ONE DOMAIN-ONE MARK” Based on pairings of PTM and their corresponding reading modules, it was assumed that a
single domain/PTM interaction can direct a complex to its genome targets because mutation of either PTM sites or recognition domains disrupts the proper recruitment of the complex. However,
accumulating evidence suggests that this one domain-one mark mechanism might not be sufficient to decipher the complex PTM language in a cellular environment. First, one PTM can be
recognized by several readers (e.g., H3K4me alone has eight different readers, Table 1). Second, complexes carrying out opposite reactions can share the same binding motif (e.g., the chromo
domain-containing Eaf3 is the subunit of histone acetyltransferase NuA4 and histone deacetylase Rpd3S 1). Third, a single domain reads several PTMs (e.g., LRWD1 within the origin recognition
complex (ORC) recognizes K9me3, K27me3 and K20me3 17). MULTIVALENT RECOGNITION Since chromatin complexes tend to contain several PTM reading modules, multivalent binding has emerged as a
prevalent theme for recognizing modified chromatin. Combinations of multiple weak interactions not only can enhance overall binding through cooperation but also allow fine-tuned regulation
of individual contacts so as to sense subtle environmental cues. We will divide our discussion into four categories. However, these mechanisms are not mutually exclusive and can be further
combined. TARGETS WITHIN ONE HISTONE The TAF1 subunit of TFIID was first reported to utilize double-bromo domains to recognize the dual-acetylated histone peptide, H4K5acK12ac 11.
Interestingly, TFIID also binds to H3K4me3 peptides (recognized by TAF3 PHD) more strongly when it is flanked by H3K9acK14ac 17, suggesting additional synergy between the PHD and bromo
domains. Similarly, the HAT-SAGA complex employs the double Tudor of the Sgf29 subunit (which binds to K4me3) and the bromo domain of Gcn5 or Spt7 (which recognizes H3K9ac14ac) to
preferentially target peptides carrying combined PTM 17. TARGETS WITHIN ONE NUCLEOSOME Multivalent recognition is more advantageous for native complexes to recognize modified nucleosomes.
The chromo domain of Eaf3 is a weak K36me reader 50. Although Eaf3 is a subunit of both NuA4 and Rpd3S, only Rpd3S binds to K36-methylated nucleosomes 51, suggesting that monovalent
recognition is not sufficient. Indeed, Rpd3S uses another reading module, the PHD domain of Rco1, in combination with the chromo domain of Eaf3 to achieve recognition of K36me in the
chromatin context 51. Likewise, the binding of the PRC2 complex to nucleosomes depends on multiple contacts: the WD40 domain of the EED subunit binds to methylated histones (Figure 3); the
N-terminus of EED interacts with histone H3 and the RbAp48 subunit binds to histone H4 52. However, it is not clear how these three independent contacts cooperatively lead to the binding of
PRC2 to the nucleosomes. TARGETS WITHIN MULTIPLE NUCLEOSOMES Multivalent recognition is also utilized by complexes to interact with an array of nucleosomes. (1) The SIR complex binds to
trinucleosomal templates through at least three contact points: Sir4 binds to DNA; Sir3 binds to the unmodified histone H4 tail; and Sir3 binds to histone H3, which is sensitive to H3K79
methylation 53. (2) PRC1 mediates chromatin compaction through its PSC subunit in a histone-tail-independent manner 54, while the chromo domain of the Pc subunit binds to H3K27-methylated
histone tails. However, whether both contacts are important for PRC1 in vivo targeting remains to be tested. (3) The DNA methyltransferase, Dnmt3b, preferentially binds to highly compacted
and hypoacetylated long nucleosomal arrays 55, suggesting that its targets likely reside in different nucleosomal surfaces. (4) Three MBT domains of L3MBTL1 can bind to at least two
nucleosomes simultaneously through the MBT/MeK interaction discussed above 38. Therefore, it is possible that L3MBTL1 brings two distant nucleosomes together – even when they are on
different chromosomes. RECOGNITION OF SPECIFIC DNA SEQUENCE AND HISTONE PTM Although most chromatin factors bind to nucleosomes regardless of underlying DNA sequence, some complexes possess
sequence-recognition ability. Besides having two PTM readers (TAF3/PHD and TAF1/bromo), TFIID also contains the TBP subunit that recognizes the TATA box and is important for TFIID template
engagement 1. The Rpd3L complex also has this combinatorial recognition potential because of the Pho23 subunit, which contains a K4me3-reading PHD domain and the Ume6/Ash1 subunits, both of
which are sequence-specific DNA-binding proteins 56. Recently, the histone demethylase, KDM2A, was shown to recognize methylated DNA 57, and it also contains a potential PTM-reading PHD
domain. REGULATION OF READING HISTONE MODICATIONS Chromatin complexes possess intrinsic properties to recognize certain PTM. However, this recognition can be regulated at two levels:
modification of the reading unit or adjustment of the signal platform. REGULATION OF THE READERS BY RNA Non-coding RNA (ncRNA) plays important roles in targeting chromatin regulators to
their cognate sites. The CBX7 subunit of the PRC1 complex not only contains a chromo domain that reads K27me2, but also recognizes an antisense ncRNA transcribed from the INK4b/ARF/INK4a
locus using a different binding surface 58. ncRNA association is important for PRC1 targeting and repression functions 58. Similarly, short ncRNA generated from PRC2-repressed promoters
forms stem-loop structures that interact with PRC2 and control its localization 59. _HOTAIR_ ncRNA and _Xist_ RNA also help targeting PRC2 _in cis_ 60, 61. In these cases, it is not clear
how RNA binding coordinates with PTM recognition. BY BINDING PARTNERS HP1 reads K9me through its chromo domain. However, it was found that ORC and HP1 are mutually required for each other to
bind to K9-methylated heterochromatin 62. Moreover, the binding of HP1 to K9-methylated nucleosomes is stimulated by addition of the auxiliary factors ACF1 and SU(VAR)3-9 63, underscoring
the importance of binding partners for PTM recognition. BY CONFORMATIONAL CHANGES OF THE READERS The tandem bromo domains of the Rsc4 subunit of the remodeling complex RSC are responsible
for recognizing H3K14ac (second bromo domain – BD2; Figure 1A, right side) 24. Interestingly, the first bromo domain also binds to acetylated K25 of Rsc4, which is sufficient to inhibit the
binding of BD2 to H3K14ac, presumably due to steric hindrance 24. This result manifests a novel auto-regulatory mechanism for PTM binding. In another example, one of four pocket-forming
residues (W364) only rotates to the proper position when EED is bound by histone peptides, suggesting that the binding site for MeK is cryptic and a conformational change driven by histone
peptide binding is required 36, 37. REGULATION OF THE SIGNAL CONTEXT BY OTHER MODIFICATIONS (HISTONE MODIFICATION CROSS-TALK) The flanking sequences make important contributions to PTM
reading, therefore, modification at adjacent residues could easily influence the binding of the readers. For instance, H3S10Ph releases the binding of HP1 to K9me 64 and phosphorylation of
H3T6 disrupts LSD1 binding to K4me 65. As for K4me readers, H3R2 binds to a different pocket from H3K4 (Figure 2A and 2B). But steric hindrance caused by addition of methyl groups in MeR
directly reduces the H3R2 binding, which in turn decreases overall affinity of K4-methylated peptides 30. Another interesting case is that of H3K4ac, which differentially regulates two K9me
readers – the Chp1/Clr4 methyltransferase complex and the Chp2/Swi6 complex – thus tipping the balance of these two important heterochromatin regulators during different cell-cycle stages
66. PTM recognition can also be regulated by histone modifications at distant residues. For instance, phosphorylation of H3Y41 inhibits HP1α binding to K9me 67. However, secondary effectors
might mediate such an effect in this case. BY CHROMATIN CONTEXT Although Crb2 (53BP1) recognizes H4K20me and H3K79me _in vitro_, those two marks are not accessible to Crb2 before DNA damage
16, 68. Therefore, changes of topological tension and/or the high-order chromatin structure upon DNA damage are important for displaying those marks to downstream readers. FUNCTIONAL
READOUTS OF PTM The functional readouts of particular PTM are dictated by functions of the effectors/readers. We will categorize these readers into four groups (Figure 6). CHROMATIN
ARCHITECTURAL PROTEINS Protein complexes that bind to multiple nucleosomes simultaneously have the potential to induce chromatin compaction or serve as physical shields to block access to
underlying DNA. These so-called architectural proteins often spread across a large region through self-propagation and oligomerization 69, such as the SIR complex (which targets
hypoacetylated and H3K79-unmethylated regions) 53 and heterochromatin protein 1 (which binds to K9me) 12. These architectural proteins can even remain bound to nucleosomes during DNA
replication, such as the PRC1 complex 70. CHROMATIN REMODELERS Once targeted by PTM, chromatin remodeling complexes either make nucleosomal DNA more accessible or mobilize nucleosomes to
different positions 4. For instance, the remodeler, RSC, targets hyperacetylated nucleosomes at coding regions 71. The BPTF subunit of NURF contains an H3K4me3-reading PHD domain and an
AcK-reading bromo domain, both of which are important for NURF localization 72. One unique feature of these readers is that they might not be enriched at their true targets because of a
hit-and-run mode of action in which remodelers do not stay bound to the region after the reaction (e.g., the yeast Isw2 complex) 73. CHROMATIN MODIFIERS Many primary PTMs do not have direct
influence on chromatin structure except for recruiting secondary chromatin modifiers that can either modify or de-modify local chromatin. For instance, K36me3 functions by recruiting Rpd3S
to deacetylate transcribed chromatin 50. K20me1 is recognized by the PWWP domain of Pdp1, thereby localizing the Set9 methyltransferase to convert K20me1 into K20me3 74. KDM4a is guided by
its Tudor domain to H3K4me3 and H4K20me3 regions to demethylate me2 and me3 on K9 and K36 75. Moreover, PTM recognition also directs DNA modifiers. DNA methyltransferase, Dnmt3a, binds to
K36me3 via its PWWP domain 76, and its partner, Dnmt3L, recognizes K4me0 32. Given the overlapping pattern of these two marks, DNA methylation could be precisely guided by histone PTM.
RECRUITMENT OF OTHER MACHINERY PTM readers can serve as adaptors to recruit factors that are directly involved in DNA metabolism activities. _Transcription_: General transcription factor,
TFIID, reads both AcK and H3K4me3 signals 17. _DNA damage repair_: MDC1 binds to phosphorylated H2AX near double-strand break (DSB)-flanking chromatin and subsequently activates a cascade of
phosphorylation events that lead to the recruitment of histone-Ub ligase, RNF8. Histone ubiquitination then either acts to recruit repair machinery or somehow exposes H4K20me and H3K79me
for 53BP1 recruitment 77. _Recombination_: Recombination-activating protein, RAG2, binds to H3K4me3 at transcribed genes while RAG1 recognizes the recombination signal sequence. Neither of
them is sufficient to initiate recombination; however, when these two signals overlap, RAG1 and RAG2 multimerize to start recombination 78. _RNA processing_: MRG15 recognizes K36me3 at
transcribed regions via its chromo domain and recruits splicing regulator PTB to control alternative splicing 79. _Replication_: Both PTM patterns and genome accessibility are important for
replication timing 80, 81, implying that DNA replication machinery also has the capacity to recognize histone modifications. Recently, it is shown that an ORC-associated protein – LRWD1 –
recognizes both DNA methylation and histone modification, and is important for initiation of DNA replication 17, 18, 82. FUTURE DIRECTIONS Reading histone modification is a highly
context-dependent process. A recent systematic protein-localization mapping reveals that the chromo domain-containing MRG15 is only recruited to a subset of K36me3-enriched genes 83,
implying that there must be another unidentified essential recruiting signal. Therefore, a general challenge for the field is to identify the preferred PTM combinations for certain chromatin
readers. In addition, little is known about readers that recognize PTM on histone globular domains. Future screens using modified nucleosomal arrays might provide useful insights in this
regard. REFERENCES * Li B, Carey M, Workman JL . The role of chromatin during transcription. _Cell_ 2007; 128:707–719. Article CAS PubMed Google Scholar * Ahmad K, Henikoff S .
Epigenetic consequences of nucleosome dynamics. _Cell_ 2002; 111:281–284. Article CAS PubMed Google Scholar * Kouzarides T . Chromatin modifications and their function. _Cell_ 2007;
128:693–705. Article CAS PubMed Google Scholar * Workman JL, Kingston RE . Alteration of nucleosome structure as a mechanism of transcriptional regulation. _Annu Rev Biochem_ 1998;
67:545–579. Article CAS PubMed Google Scholar * Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL . Histone H4-K16 acetylation controls chromatin structure and protein
interactions. _Science_ 2006; 311:844–847. Article CAS PubMed Google Scholar * Jenuwein T, Allis CD . Translating the histone code. _Science_ 2001; 293:1074–1080. Article CAS PubMed
Google Scholar * Strahl BD, Allis CD . The language of covalent histone modifications. _Nature_ 2000; 403:41–45. Article CAS PubMed Google Scholar * Taverna SD, Li H, Ruthenburg AJ,
Allis CD, Patel DJ . How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. _Nat Struct Mol Biol_ 2007; 14:1025–1040. Article CAS PubMed
PubMed Central Google Scholar * Berger SL . The complex language of chromatin regulation during transcription. _Nature_ 2007; 447:407–412. Article CAS PubMed Google Scholar * Dhalluin
C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM . Structure and ligand of a histone acetyltransferase bromodomain. _Nature_ 1999; 399:491–496. Article CAS PubMed Google Scholar *
Jacobson RH, Ladurner AG, King DS, Tjian R . Structure and function of a human TAFII250 double bromodomain module. _Science_ 2000; 288:1422–1425. Article CAS PubMed Google Scholar *
Bannister AJ, Zegerman P, Partridge JF, _et al_. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. _Nature_ 2001; 410:120–124. Article CAS PubMed Google
Scholar * Kim J, Daniel J, Espejo A, _et al_. Tudor, MBT and chromo domains gauge the degree of lysine methylation. _EMBO Rep_ 2006; 7:397–403. CAS PubMed PubMed Central Google Scholar
* Bua DJ, Kuo AJ, Cheung P, _et al_. Epigenome microarray platform for proteome-wide dissection of chromatin-signaling networks. _PLoS One_ 2009; 4:e6789. Article CAS PubMed PubMed
Central Google Scholar * Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP . MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA
double-strand breaks. _Cell_ 2005; 123:1213–1226. Article CAS PubMed Google Scholar * Huyen Y, Zgheib O, Ditullio RA Jr, _et al_. Methylated lysine 79 of histone H3 targets 53BP1 to DNA
double-strand breaks. _Nature_ 2004; 432:406–411. Article CAS PubMed Google Scholar * Vermeulen M, Eberl HC, Matarese F, _et al_. Quantitative interaction proteomics and genome-wide
profiling of epigenetic histone marks and their readers. _Cell_ 2010; 142:967–980. Article CAS PubMed Google Scholar * Bartke T, Vermeulen M, Xhemalce B, Robson SC, Mann M, Kouzarides T
. Nucleosome-interacting proteins regulated by DNA and histone methylation. _Cell_ 2010; 143:470–484. Article CAS PubMed PubMed Central Google Scholar * Shahbazian MD, Grunstein M .
Functions of site-specific histone acetylation and deacetylation. _Annu Rev Biochem_ 2007; 76:75–100. Article CAS PubMed Google Scholar * Dion MF, Altschuler SJ, Wu LF, Rando OJ .
Genomic characterization reveals a simple histone H4 acetylation code. _Proc Natl Acad Sci USA_ 2005; 102:5501–5506. Article CAS PubMed PubMed Central Google Scholar * Lee KK, Workman
JL . Histone acetyltransferase complexes: one size doesn't fit all. _Nat Rev Mol Cell Biol_ 2007; 8:284–295. Article CAS PubMed Google Scholar * Lange M, Kaynak B, Forster UB, _et
al_. Regulation of muscle development by DPF3, a novel histone acetylation and methylation reader of the BAF chromatin remodeling complex. _Genes Dev_ 2008; 22:2370–2384. Article CAS
PubMed PubMed Central Google Scholar * Zeng L, Zhang Q, Li S, Plotnikov AN, Walsh MJ, Zhou MM . Mechanism and regulation of acetylated histone binding by the tandem PHD finger of DPF3b.
_Nature_ 2010; 466:258–262. Article CAS PubMed PubMed Central Google Scholar * VanDemark AP, Kasten MM, Ferris E, Heroux A, Hill CP, Cairns BR . Autoregulation of the rsc4 tandem
bromodomain by gcn5 acetylation. _Mol Cell_ 2007; 27:817–828. Article CAS PubMed PubMed Central Google Scholar * Moriniere J, Rousseaux S, Steuerwald U, _et al_. Cooperative binding of
two acetylation marks on a histone tail by a single bromodomain. _Nature_ 2009; 461:664–668. Article CAS PubMed Google Scholar * Cairns BR, Schlichter A, Erdjument-Bromage H, Tempst P,
Kornberg RD, Winston F . Two functionally distinct forms of the RSC nucleosome-remodeling complex, containing essential AT hook, BAH, and bromodomains. _Mol Cell_ 1999; 4:715–723. Article
CAS PubMed Google Scholar * Charlop-Powers Z, Zeng L, Zhang Q, Zhou MM . Structural insights into selective histone H3 recognition by the human Polybromo bromodomain 2. _Cell Res_ 2010;
20:529–538. Article CAS PubMed Google Scholar * Tsukada Y, Fang J, Erdjument-Bromage H, _et al_. Histone demethylation by a family of JmjC domain-containing proteins. _Nature_ 2006;
439:811–816. Article CAS PubMed Google Scholar * Vermeulen M, Mulder KW, Denissov S, _et al_. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. _Cell_
2007; 131:58–69. Article CAS PubMed Google Scholar * van Ingen H, van Schaik FMA, Wienk H, _et al_. Structural insight into the recognition of the H3K4me3 mark by the TFIID subunit
TAF3. _Structure_ 2008; 16:1245–1256. Article CAS PubMed Google Scholar * Lan F, Collins RE, De Cegli R, _et al_. Recognition of unmethylated histone H3 lysine 4 links BHC80 to
LSD1-mediated gene repression. _Nature_ 2007; 448:718–722. Article CAS PubMed PubMed Central Google Scholar * Ooi SK, Qiu C, Bernstein E, _et al_. DNMT3L connects unmethylated lysine 4
of histone H3 to _de novo_ methylation of DNA. _Nature_ 2007; 448:714–717. Article CAS PubMed PubMed Central Google Scholar * Org T, Chignola F, Hetenyi C, _et al_. The autoimmune
regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. _EMBO Rep_ 2008; 9:370–376. Article CAS PubMed PubMed Central Google Scholar * Wang Y, Jia S .
Degrees make all the difference: the multifunctionality of histone H4 lysine 20 methylation. _Epigenetics_ 2009; 4:273–276. Article CAS PubMed Google Scholar * Li B, Jackson J, Simon MD,
_et al_. Histone H3 lysine 36 dimethylation (H3K36me2) is sufficient to recruit the Rpd3s histone deacetylase complex and to repress spurious transcription. _J Biol Chem_ 2009;
284:7970–7976. Article CAS PubMed PubMed Central Google Scholar * Margueron R, Justin N, Ohno K, _et al_. Role of the polycomb protein EED in the propagation of repressive histone
marks. _Nature_ 2009; 461:762–767. Article CAS PubMed PubMed Central Google Scholar * Xu C, Bian C, Yang W, _et al_. Binding of different histone marks differentially regulates the
activity and specificity of polycomb repressive complex 2 (PRC2). _Proc Natl Acad Sci USA_ 2010; 107:19266–19271. Article CAS PubMed PubMed Central Google Scholar * Trojer P, Li G, Sims
RJ 3rd, _et al_. L3MBTL1, a histone-methylation-dependent chromatin lock. _Cell_ 2007; 129:915–928. Article CAS PubMed Google Scholar * Zhao Q, Rank G, Tan YT, _et al_. PRMT5-mediated
methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. _Nat Struct Mol Biol_ 2009; 16:304–311. Article CAS PubMed PubMed Central Google
Scholar * Yang Y, Lu Y, Espejo A, _et al_. TDRD3 is an effector molecule for arginine-methylated histone marks. _Mol Cell_ 2010; 40:1016–1023. Article CAS PubMed PubMed Central Google
Scholar * Kirmizis A, Santos-Rosa H, Penkett CJ, Singer MA, Green RD, Kouzarides T . Distinct transcriptional outputs associated with mono- and dimethylated histone H3 arginine 2. _Nat
Struct Mol Biol_ 2009; 16:449–451. Article CAS PubMed PubMed Central Google Scholar * Jin J, Cai Y, Li B, _et al_. In and out: histone variant exchange in chromatin. _Trends Biochem
Sci_ 2005; 30:680–687. Article CAS PubMed Google Scholar * Macdonald N, Welburn JP, Noble ME, _et al_. Molecular basis for the recognition of phosphorylated and phosphoacetylated histone
H3 by 14-3–3. _Mol Cell_ 2005; 20:199–211. Article CAS PubMed Google Scholar * Walter W, Clynes D, Tang Y, Marmorstein R, Mellor J, Berger SL . 14-3-3 interaction with histone H3
involves a dual modification pattern of phosphoacetylation. _Mol Cell Biol_ 2008; 28:2840–2849. Article CAS PubMed PubMed Central Google Scholar * Winget JM, Mayor T . The diversity of
ubiquitin recognition: hot spots and varied specificity. _Mol Cell_ 2010; 38:627–635. Article CAS PubMed Google Scholar * Geng F, Tansey WP . Polyubiquitylation of histone H2B. _Mol Biol
Cell_ 2008; 19:3616–3624. Article CAS PubMed PubMed Central Google Scholar * Lee JS, Shukla A, Schneider J, _et al_. Histone crosstalk between H2B monoubiquitination and H3 methylation
mediated by COMPASS. _Cell_ 2007; 131:1084–1096. Article CAS PubMed Google Scholar * Zheng S, Wyrick JJ, Reese JC . Novel trans-tail regulation of H2B ubiquitylation and H3K4
methylation by the N terminus of histone H2A. _Mol Cell Biol_ 2010; 30:3635–3645. Article CAS PubMed PubMed Central Google Scholar * Lu X, Simon MD, Chodaparambil JV, Hansen JC, Shokat
KM, Luger K . The effect of H3K79 dimethylation and H4K20 trimethylation on nucleosome and chromatin structure. _Nat Struct Mol Biol_ 2008; 15:1122–1124. Article CAS PubMed PubMed Central
Google Scholar * Carrozza MJ, Li B, Florens L, _et al_. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription.
_Cell_ 2005; 123:581–592. Article CAS PubMed Google Scholar * Li B, Gogol M, Carey M, Lee D, Seidel C, Workman JL . Combined action of PHD and chromo domains directs the Rpd3S HDAC to
transcribed chromatin. _Science_ 2007; 316:1050–1054. Article CAS PubMed Google Scholar * Murzina NV, Pei XY, Zhang W, _et al_. Structural basis for the recognition of histone H4 by the
histone-chaperone RbAp46. _Structure_ 2008; 16:1077–1085. Article CAS PubMed PubMed Central Google Scholar * Martino F, Kueng S, Robinson P, _et al_. Reconstitution of yeast silent
chromatin: multiple contact sites and O-AADPR binding load SIR complexes onto nucleosomes _in vitro_. _Mol Cell_ 2009; 33:323–334. Article CAS PubMed Google Scholar * Francis NJ,
Kingston RE, Woodcock CL . Chromatin compaction by a polycomb group protein complex. _Science_ 2004; 306:1574–1577. Article CAS PubMed Google Scholar * Kashiwagi K, Nimura K, Ura K,
Kaneda Y . DNA methyltransferase 3b preferentially associates with condensed chromatin. _Nucleic Acids Res_ 2011; 39:874–888. Article CAS PubMed Google Scholar * Carrozza MJ, Florens L,
Swanson SK, _et al_. Stable incorporation of sequence specific repressors Ash1 and Ume6 into the Rpd3L complex. _Biochim Biophys Acta_ 2005; 1731:77–87; discussion 75–76. Article CAS
PubMed Google Scholar * Blackledge NP, Zhou JC, Tolstorukov MY, Farcas AM, Park PJ, Klose RJ . CpG islands recruit a histone H3 lysine 36 demethylase. _Mol Cell_ 2010; 38:179–190. Article
CAS PubMed PubMed Central Google Scholar * Yap KL, Li S, Munoz-Cabello AM, _et al_. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7
in transcriptional silencing of INK4a. _Mol Cell_ 2010; 38:662–674. Article CAS PubMed PubMed Central Google Scholar * Kanhere A, Viiri K, Araujo CC, _et al_. Short RNAs are transcribed
from repressed polycomb target genes and interact with polycomb repressive complex-2. _Mol Cell_ 2010; 38:675–688. Article CAS PubMed PubMed Central Google Scholar * Tsai MC, Manor O,
Wan Y, _et al_. Long noncoding RNA as modular scaffold of histone modification complexes. _Science_ 2010; 329:689–693. Article CAS PubMed PubMed Central Google Scholar * Zhao J, Sun BK,
Erwin JA, Song JJ, Lee JT . Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. _Science_ 2008; 322:750–756. Article CAS PubMed PubMed Central Google Scholar *
Prasanth SG, Shen Z, Prasanth KV, Stillman B . Human origin recognition complex is essential for HP1 binding to chromatin and heterochromatin organization. _Proc Natl Acad Sci USA_ 2010;
107:15093–15098. Article CAS PubMed PubMed Central Google Scholar * Eskeland R, Eberharter A, Imhof A . HP1 binding to chromatin methylated at H3K9 is enhanced by auxiliary factors.
_Mol Cell Biol_ 2007; 27:453–465. Article CAS PubMed Google Scholar * Fischle W, Tseng BS, Dormann HL, _et al_. Regulation of HP1-chromatin binding by histone H3 methylation and
phosphorylation. _Nature_ 2005; 438:1116–1122. Article CAS PubMed Google Scholar * Metzger E, Imhof A, Patel D, _et al_. Phosphorylation of histone H3T6 by PKCbeta(I) controls
demethylation at histone H3K4. _Nature_ 2010; 464:792–796. Article CAS PubMed Google Scholar * Xhemalce B, Kouzarides T . A chromodomain switch mediated by histone H3 Lys 4 acetylation
regulates heterochromatin assembly. _Genes Dev_ 2010; 24:647–652. Article CAS PubMed PubMed Central Google Scholar * Dawson MA, Bannister AJ, Gottgens B, _et al_. JAK2 phosphorylates
histone H3Y41 and excludes HP1alpha from chromatin. _Nature_ 2009; 461:819–822. Article CAS PubMed PubMed Central Google Scholar * Botuyan MV, Lee J, Ward IM, _et al_. Structural basis
for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. _Cell_ 2006; 127:1361–1373. Article CAS PubMed PubMed Central Google Scholar * Buhler
M, Gasser SM . Silent chromatin at the middle and ends: lessons from yeasts. _EMBO J_ 2009; 28:2149–2161. Article CAS PubMed PubMed Central Google Scholar * Francis NJ, Follmer NE,
Simon MD, Aghia G, Butler JD . Polycomb proteins remain bound to chromatin and DNA during DNA replication _in vitro_. _Cell_ 2009; 137:110–122. Article CAS PubMed PubMed Central Google
Scholar * Carey M, Li B, Workman JL . RSC Exploits Histone Acetylation to Abrogate the Nucleosomal Block to RNA Polymerase II Elongation. _Mol Cell_ 2006; 24:481–487. Article CAS PubMed
PubMed Central Google Scholar * Ruthenburg AJ, Li H, Patel DJ, Allis CD . Multivalent engagement of chromatin modifications by linked binding modules. _Nat Rev Mol Cell Biol_ 2007;
8:983–994. Article CAS PubMed PubMed Central Google Scholar * Gelbart ME, Bachman N, Delrow J, Boeke JD, Tsukiyama T . Genome-wide identification of Isw2 chromatin-remodeling targets by
localization of a catalytically inactive mutant. _Gene Dev_ 2005; 19:942–954. Article CAS PubMed PubMed Central Google Scholar * Wang Y, Reddy B, Thompson J, _et al_. Regulation of
Set9-mediated H4K20 methylation by a PWWP domain protein. _Mol Cell_ 2009; 33:428–437. Article CAS PubMed PubMed Central Google Scholar * Lan F, Nottke AC, Shi Y . Mechanisms involved
in the regulation of histone lysine demethylases. _Curr Opin Cell Biol_ 2008; 20:316–325. Article CAS PubMed PubMed Central Google Scholar * Dhayalan A, Rajavelu A, Rathert P, _et al_.
The Dnmt3a PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation. _J Biol Chem_ 2010; 285:26114–26120. Article CAS PubMed PubMed Central Google Scholar *
Jungmichel S, Stucki M . MDC1: The art of keeping things in focus. _Chromosoma_ 2010; 119:337–349. Article CAS PubMed Google Scholar * Ji Y, Resch W, Corbett E, Yamane A, Casellas R,
Schatz DG . The _in vivo_ pattern of binding of RAG1 and RAG2 to antigen receptor loci. _Cell_ 2010; 141:419–431. Article CAS PubMed PubMed Central Google Scholar * Luco RF, Pan Q,
Tominaga K, Blencowe BJ, Pereira-Smith OM, Misteli T . Regulation of alternative splicing by histone modifications. _Science_ 2010; 327:996–1000. Article CAS PubMed PubMed Central Google
Scholar * Bell O, Schwaiger M, Oakeley EJ, _et al_. Accessibility of the _Drosophila_ genome discriminates PcG repression, H4K16 acetylation and replication timing. _Nat Struct Mol Biol_
2010; 17:894–900. Article CAS PubMed Google Scholar * Vogelauer M, Rubbi L, Lucas I, Brewer BJ, Grunstein M . Histone acetylation regulates the time of replication origin firing. _Mol
Cell_ 2002; 10:1223–1233. Article CAS PubMed Google Scholar * Shen Z, Sathyan KM, Geng Y, _et al_. A WD-repeat protein stabilizes ORC binding to chromatin. _Mol Cell_ 2010; 40:99–111.
Article CAS PubMed PubMed Central Google Scholar * Filion GJ, van Bemmel JG, Braunschweig U, _et al_. Systematic protein location mapping reveals five principal chromatin types in
_Drosophila_ cells. _Cell_ 2010; 143:212–224. Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We are indebted to Dr Chun Ruan (UT Southwestern
Medical Center) for her critical comments on the manuscript and Dr Carson Harrod (Baylor Institute for Immunology Research) for editorial assistance. We also thank Phi Luong (UT Southwestern
Medical Center) for his help in preparing the figures. BL is a W.A. “Tex” Moncrief, Jr Scholar in Medical Research and supported by grants from the National Institutes of Health
(R01GM090077), the Welch Foundation (I-1713), March of Dimes Foundation and the American Heart Association. JLW is supported by Stowers Institute for Medical Research. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Department of Molecular Biology, UT Southwestern Medical Center, Dallas, 75390-9148, TX, USA Miyong Yun, Jun Wu & Bing Li * Stowers Medical Research Institute,
Kansas City, 64110, MO, USA Jerry L Workman Authors * Miyong Yun View author publications You can also search for this author inPubMed Google Scholar * Jun Wu View author publications You
can also search for this author inPubMed Google Scholar * Jerry L Workman View author publications You can also search for this author inPubMed Google Scholar * Bing Li View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Bing Li. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Yun, M., Wu, J., Workman, J. _et al._ Readers of histone modifications. _Cell Res_ 21, 564–578 (2011). https://doi.org/10.1038/cr.2011.42 Download citation * Published: 22
March 2011 * Issue Date: April 2011 * DOI: https://doi.org/10.1038/cr.2011.42 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * histone
modification * chromatin * epigenetics