Spermidine promotes stress resistance in drosophila melanogaster through autophagy-dependent and -independent pathways
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The naturally occurring polyamine spermidine (Spd) has recently been shown to promote longevity across species in an autophagy-dependent manner. Here, we demonstrate that Spd
improves both survival and locomotor activity of the fruit fly _Drosophila melanogaster_ upon exposure to the superoxide generator and neurotoxic agent paraquat. Although survival to a high
paraquat concentration (20 mM) was specifically increased in female flies only, locomotor activity and survival could be rescued in both male and female animals when exposed to lower
paraquat levels (5 mM). These effects are dependent on the autophagic machinery, as Spd failed to confer resistance to paraquat-induced toxicity and locomotor impairment in flies deleted for
the essential autophagic regulator ATG7 (autophagy-related gene 7). Spd treatment did also protect against mild doses of another oxidative stressor, hydrogen peroxide, but in this case in
an autophagy-independent manner. Altogether, this study establishes that the protective effects of Spd can be exerted through different pathways that depending on the oxidative stress
scenario do or do not involve autophagy. SIMILAR CONTENT BEING VIEWED BY OTHERS MOLECULAR MECHANISMS OF EXCEPTIONAL LIFESPAN INCREASE OF _DROSOPHILA MELANOGASTER_ WITH DIFFERENT GENOTYPES
AFTER COMBINATIONS OF PRO-LONGEVITY INTERVENTIONS Article Open access 09 June 2022 PREVENTING EXCESSIVE AUTOPHAGY PROTECTS FROM THE PATHOLOGY OF MTDNA MUTATIONS IN _DROSOPHILA MELANOGASTER_
Article Open access 23 December 2024 AUTOPHAGY: A NECESSARY DEFENSE AGAINST EXTREME CADMIUM INTOXICATION IN A MULTIGENERATIONAL 2D EXPERIMENT Article Open access 03 December 2020 MAIN The
population’s proportion of older people is steadily increasing in many countries and with it the incidence of experiencing the process of ageing. More people will live longer1 but will also
have an elevated risk of suffering age-associated disabilities and diseases. Thus, being able to postpone and/or lessen the deleterious effects of ageing represents an acute challenge for
modern society and would bring high societal and economical advantages. Our understanding of ageing has increased at an unprecedented pace during the last 30 years and we have now realised
that ageing is a plastic process that can be modulated. Indeed, longevity is partly under genetic control and mutations in single genes have been shown to increase life span and,
interestingly, also stress resistance in model organisms.2 Alternatively, ageing can be modulated by external, non-genetic interventions. One of them is dietary restriction, where a decrease
in the amount of food intake delays ageing, disease onset and mortality in a wide range of organisms, including non-human primates.3 Furthermore, several pharmacological interventions have
been recently reported to be beneficial for ageing, diseases and life span extension, even though the obtained results do not offer a clear picture. For instance, resveratrol, a naturally
occurring phenol found, for example, in the skin of red grapes, increased the life span of mice kept on a high-fat diet.4 However, the doses used to achieve life span extension were very
high, raising the question of this compound’s bioavailability. In addition, resveratrol has not been shown to exert any beneficial effect in healthy organisms.5 The immunosuppressant drug
rapamycin has also been demonstrated to increase the life span of rodents5, 6 but only shows inconclusive effects in the fruit fly _Drosophila melanogaster._7, 8 Notably, a serious drawback
of rapamycin is its immunosuppressant properties, which could hinder its potential use on a wide basis in healthy organisms. Altogether, these examples clearly show that further research is
needed to broaden our knowledge on the effects and mechanisms governing the activity of already identified molecules and to find new ones. Spermidine (Spd) is a natural polyamine involved in
an array of crucial molecular processes such as DNA stability, transcription, translation, apoptosis, cell proliferation, differentiation and survival. Intriguingly, its intracellular level
decreases with age.9, 10 We have recently shown that addition of Spd to the food medium increases the life span of yeast, worms, flies and the survival of human immune cells in culture.11
Spd also reduced age-related oxidative damage in mice and increased resistance to hydrogen peroxide (H2O2) and heat in yeast. We further showed that Spd induced intracellular self-digestion
(autophagy) to exert its life span extension effect, which could be abrogated by genetic inactivation of autophagy genes in mutant yeast, worms and flies. The regulatory mechanism underlying
this effect might be of epigenetic origin. We observed that in yeast, Spd inhibits histone acetyltransferases activity and leads to a global hypoacetylation of histone H3 at all acetylation
sites located at the amino terminus of the histone. Consistent with the anti-ageing potential of Spd, its intracellular reduction decreased the life span of mice.12 Accordingly, in a
further report external feeding with polyamines increased life span and reduced age-associated pathology in a short-lived mouse model.13 However, the latter results need to be confirmed as
the study was stopped when a significant amount of mice were still alive (after 88 weeks of age). Taken together, these results suggest that Spd could be a powerful tool against the
deleterious consequences of ageing.14 Anti-ageing properties are often correlated with high stress resistance.15, 16 In the present study, we address the effects of Spd on two
ageing-relevant stresses, oxidative stress and starvation, in the fruit fly _D. melanogaster_. To this end, we first challenged flies with the herbicide paraquat, which is a neurotoxic agent
widely employed to generate oxidative stress through the reactive oxygen species superoxide. We show that upon paraquat exposure, treatment with Spd improves survival in female flies
exposed to high paraquat levels (20 mM). In addition, it confers longer retention of locomotor activity and survival in both male and female animals when challenged with a lower paraquat
concentration (5 mM). We also demonstrate that these effects are exerted in an autophagy-dependent manner. Spd also increases resistance to a different oxidative stressor, H2O2, when applied
at mild doses (1%). In contrast to paraquat, however, this resistance is not dependent upon functional autophagy, hinting to differential pathways governing Spd-mediated resistance to
different oxidative stressors. Finally, we show that Spd fails to rescue toxicity induced by more severe H2O2 levels (2%) or starvation. These results suggest that Spd protects against
toxicity resulting from detrimental pathways that involve selective oxidative stress. Furthermore, this protection can be exerted via different mechanisms (that do or do not involve
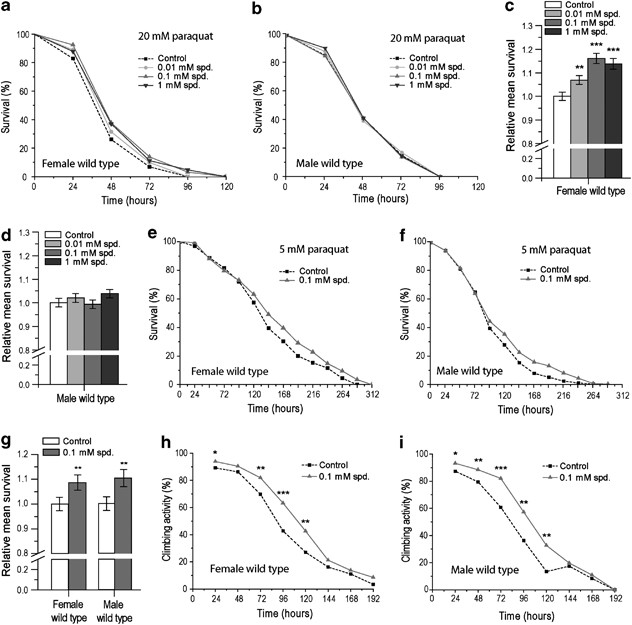
autophagy) depending on the oxidative stress scenario. RESULTS SPD IMPROVES SURVIVAL DURING 20 MM PARAQUAT STRESS IN FEMALE _DROSOPHILA_ Paraquat is a superoxide generator that has been
used, for instance, as a neurotoxic agent to model age-related neurodegenerative diseases in the fruit fly _D. melanogaster_.17 To test the oxidative stress response upon Spd treatment,
flies were exposed to 20 mM paraquat and either fed Spd or left untreated. As expected, paraquat strongly compromised fly survival, killing 50% of the untreated animals (males and females)
in average after approximately 55 h of exposure (Figures 1a and b). When treated with Spd, female flies challenged with paraquat displayed a significantly better mean and maximum survival,
reaching the overall largest improvement at a Spd concentration of 0.1 mM (Figures 1a and c). In males on the other hand, the additional treatment with Spd did not influence paraquat-induced
toxicity over the tested concentration range (Figures 1b and d). For variance between replicates, refer to the Supplementary Material (Supplementary Figures S1A, B). These data suggest that
Spd confers sex-specific paraquat resistance, specifically favoring survival in female flies. SPD INCREASES SURVIVAL AND CLIMBING ACTIVITY DURING 5 MM PARAQUAT EXPOSURE IN BOTH SEXES In
addition to detrimental oxidative stress, paraquat also induces locomotor impairment. Thus, we next decided to determine whether Spd might influence locomotor activity upon paraquat
exposure. For this purpose, we monitored the ability of flies to climb the vertical wall of the vial in which they were kept, until no fly could perform the task. To decelerate the incidence
of death and follow their locomotor activity for a longer period of time, flies were exposed to a lower concentration of paraquat compared to the above survival experiments (5 mM instead of
20 mM). Indeed, this concentration allowed prolonged overall survival compared to 20 mM (compare Figures 1a and b with Figures 1e and f). In addition, treatment with 0.1 mM Spd – the
concentration having shown the biggest survival increase to 20 mM paraquat – improved survival to paraquat, and this time in both sexes (Figures 1e–g, Supplementary Figures S1C, D).
Similarly, the climbing activities of both female and male flies fed with 0.1 mM Spd was improved compared with the untreated control (Figures 1h and i, Supplementary Figures S1E–J). At 96 h
after the beginning of paraquat exposure, for instance, Spd-treated females displayed about 30% higher activity than control flies (Figure 1h). In male flies, similar rates were obtained
(Figure 1i). Altogether, these data demonstrate that Spd can lessen death, as well as locomotor impairment in both male and female flies, upon exposure to low but toxic paraquat doses (5
mM). SPD-IMPROVED SURVIVAL AND CLIMBING ACTIVITY ARE DEPENDENT ON AUTOPHAGY Spd-mediated survival improvement during ageing is at least partly associated with autophagy,11 a self-digestion
mechanism that has been connected to longevity in various organisms.18 To test if this process is involved in Spd-induced paraquat resistance, we performed survival and climbing activity
experiments in loss-of-function mutants for atg7 (autophagy-related gene 7) (_atg7__–/–_), a gene essential for autophagy. In contrast to the results obtained in wild-type flies, in
autophagy mutants Spd treatment did not improve the survival decrease caused by paraquat exposure, neither in females with 20 mM nor in both sexes with 5 mM paraquat (Figures 2a–g,
Supplementary Figures S2A–D). In fact, in the autophagy-depleted background, Spd treatment even seemed to decrease female survival as compared with the control (Figure 2c, Supplementary
Figure S2A). Similarly, paraquat-induced locomotor impairment could not be rescued neither in female nor male atg7-deficient animals (Figures 2h and i, Supplementary Figures S2E–J) as
opposed to the results obtained in wild-type flies (compare with Figures 1h and i). Thus, autophagy is essential for Spd-mediated resistance to paraquat-induced toxicity as well as for
paraquat-induced loss of locomotor performance. SPD CONFERS RESISTANCE TO MILD H2O2 EXPOSURE BUT NOT TO MORE SEVERE EXPOSURE OR TO STARVATION Given that paraquat specifically induces the
generation of superoxide anion radicals, we asked whether the observed rescuing effect of Spd was the result of a protection against paraquat-specific or rather general oxidative stress. To
test this, we examined survival of wild-type male and female flies in a different oxidative stress situation, namely upon exposure to H2O2. As observed with 5 mM paraquat, the decrease in
survival resulting from challenge to 1% H2O2 could be partly rescued by Spd supplementation in both male and female flies (Figures 3a, b and e). However, in contrast to paraquat exposure,
Spd treatment also increased survival to 1% H2O2 in autophagy-deficient atg7−/− flies (Figures 3c, d and f). In turn, when H2O2 was applied at a higher concentration (2%), Spd
supplementation could not revert the adverse effects on survival (Supplementary Figures S3A–D). Similar results were obtained in the atg7-deletion background (Supplementary Figures S3E–H).
Spd at the highest concentration even seemed to decrease survival in atg7−/− males (Supplementary Figure S3H). To evaluate if Spd might also exhibit a rescuing effect upon exposure to
adverse conditions requiring different mechanisms than oxidative stress, we starved flies by keeping them in vials containing only water and agar with or without Spd and measured survival.
Starvation stress resulted in a severe loss of viability over time reaching complete death of the population after approximately 160 and 120 h in female and male flies, respectively
(Supplementary Figures S4A, B). These survival rates remained unchanged also when flies were additionally fed Spd (Supplementary Figures S4A–F). Altogether, these data suggest that Spd
specifically reduces oxidative stress as generated through paraquat or mild doses of H2O2 but does not protect against exposure to severe H2O2 or starvation stress. DISCUSSION We have
previously shown that the polyamine Spd increases life span in various model organisms, as well as in human immune cells.11, 19, 20, 21 Life span extension has often been linked to a
concomitant increase in stress resistance. For instance, the first longevity mutant identified, the _age-1_ mutant in _Caenorhabditis elegans_, also displayed heat resistance.22 More
recently, flies mutants in _Loco_, a _Drosophila_ regulator of G-protein signaling protein, were shown to exhibit a longer life span accompanied by a higher resistance to starvation, heat
and paraquat.23 Such coupling of longevity and stress resistance is not only observed when life span is increased by mutations but also when achieved by non-genetic interventions. For
instance, dietary restriction – a well-known longevity inductor – has been demonstrated to increase stress resistance24 and decrease oxidative damage,25 although some negative effects of
dietary restriction on stress resistance have been reported too.26 Similarly, we could show that life span-extending Spd administration increases resistance to heat and H2O2 in yeast, and
decreases age-related oxidative stress in mice.11 Indeed, extensive evidence – especially in plants – supports the concept that polyamines improve stress resistance.21 In the present study,
we show that Spd confers resistance to paraquat and mild doses of H2O2 in _D. melanogaster_. Although Spd increased survival of both female and male flies upon exposure to 1% H2O2 and 5 mM
paraquat, it did so only in females when challenged to a high paraquat concentration (20 mM). In fact, it is generally observed that female flies are more resistant to stress than male
flies, most likely because females are bigger and can withstand the stress to which they are exposed longer before dying. Thus, 20 mM paraquat (in contrast to 5 mM) might be too high of a
concentration to withstand for the males but not for the females, allowing only the latter to take advantage of Spd supplementation. Of note, the big difference in size probably explains why
we observed the biggest survival difference between sexes on starvation resistance as compared with the other tested stresses: starvation resistance relies entirely on the available bodily
reserves of the organism, which in females are larger than in males. Paraquat is a superoxide generator and thus its toxicity seems to be related at least in part to oxidative damage. This
idea is supported by the fact that pure polyphenols, known for their antioxidant properties, increase survival and locomotion in flies exposed to paraquat.27 Resveratrol on the other hand,
another known antioxidant with life span-extending effects, does not rescue paraquat-induced locomotor impairment in flies and even decreases exploratory locomotion in flies not exposed to
paraquat.17 Thus, establishing the specific mechanism(s) underlying the beneficial effects of Spd on both locomotion and survival upon toxic exposure to paraquat is crucial. According to our
results, it may involve autophagy, an intracellular self-digestion process that we have previously shown to determine Spd-mediated life span extension in yeast, worms and flies.11 The
present study reveals that upon paraquat exposure the survival and the locomotor activity of autophagy-deficient mutant flies cannot be improved by Spd feeding. Autophagy is thus an
essential component of Spd-mediated resistance to paraquat. This aligns with reports showing that paraquat exposure induces autophagy as a protective response in models as diverse as the
plant _Arabidopsis_28 or human neuroblastoma cells29 and seems to be protective. In neuroblastoma cells, inhibition of autophagy accelerates apoptotic cell death, whereas in _Arabidopsis_,
the proteins oxidized by paraquat are degraded by autophagy. It thus appears that autophagy contributes to the faster or more efficient degradation of damaged molecules arising from paraquat
exposure conferring longer protection to this stress. It is tempting to speculate that a similar mechanism might underlie Spd-mediated protection against paraquat in flies, which will need
to be tested in future studies. However, autophagy does not seem to be the only mechanism by which Spd is able to confer protection in a given oxidative stress scenario. Interestingly, our
data show that while Spd supplementation also increases resistance to a different oxidative stress than paraquat, namely 1% H2O2, in this scenario Spd still increases survival in atg7−/−
flies. Thus, under H2O2 conditions autophagy does not seem to be involved in the rescuing mechanism. Interestingly, Spd seems to exhibit both autophagy-dependent but also -independent
protective effects under different conditions in yeast as well (early and late chronological aging).10, 11 The discrepancy in the effect of Spd on resistance to paraquat and H2O2 in flies
may be due to the fact that oxidative stress as induced by paraquat, which directly generates superoxide anion radicals, may work by a different mechanism than that triggered by H2O2.
Girardot _et al._30 reported that almost 10 times as many genes were up or downregulated in _Drosophila_ during paraquat compared with H2O2 exposure, although both treatments induced the
same mortality. Furthermore, some sets of genes were specific to one treatment, supporting the fact that toxicity mechanisms are different for paraquat and H2O2. For instance, 73% of the
genes encoding the 26S proteasome subunits were induced by paraquat but not by H2O2 treatment. In contrast, ubiquitin protein ligases were under-represented in genes affected by paraquat.
The authors hypothesized that _Drosophila_ can implement two types of response to oxidative stress: one relying on post-transcriptional mechanisms as induced by H2O2 and the other one
supported by a coordinated increase of proteasome genes as induced by paraquat. Of note, Spd failed to rescue toxicity resulting from more severe H2O2 exposure (2%). This may be due to the
fact that the cellular damage inflicted by very high H2O2 concentrations differs from that resulting from more moderate toxic levels. It should be noted that in this study autophagy
dependency was assayed using atg7−/− flies, which are deficient in the essential autophagic regulator ATG7. It has been shown that atg7−/− flies are generally shorter-lived, less resistant
to diverse stresses (including 30 mM paraquat, 1% H2O2 – both mixed in food – and starvation), display faster decline of climbing activity and enhanced neurodegeneration.31 Likewise, we have
previously reported that atg7−/− flies are shorter-lived.11 Our herein presented results furthermore show that lack of autophagy decreases overall survival and climbing activity upon 5 mM
paraquat exposure (albeit not always significantly) and survival to 1 and 2% H2O2. In contrast, some of our results also indicate that lack of autophagy may not always be detrimental. For
instance, overall survival to 20 mM paraquat exposure is not affected in atg7−/− animals. In fact, lack of autophagy has been already reported to not always lead to dramatic effects; for
example, atg7−/− flies do not show any developmental defect and are fully viable.31 It may also be that in our study the stress under which ATG7 deletion did not influence overall survival
(20 mM paraquat) was comparatively strong so that the lack of autophagy could not worsen an already low survival. Finally, we report that the rescuing effect of Spd as observed towards
paraquat and 1% H2O2 toxicity is absent upon challenge to a further stressor, starvation. The varying degrees of correlations reported between oxidative stress and starvation resistance in
long-lived organisms suggest that the machinery involved in executing a response to these two stress factors may or may not overlap depending on the conditions. For example, although _Loco_
mutant female flies were more resistant to both paraquat and starvation,23 Gr63a (a CO2 sensor) female mutant flies were more resistant to paraquat but not to starvation.32 Resistance to
starvation is mainly controlled by the amount of reserves (lipids and glycogen) of the flies. Thus, metabolic changes induced by Spd supplementation may prevent beneficial effects of Spd on
survival to this stress. Although it is yet unknown whether Spd does alter metabolism, some evidence hints towards it. For instance, mice fed a high polyamine diet have been reported to be
hyperphagic.13 Current work studying the effect of Spd on metabolism in _Drosophila_ will help to clarify a putative role of Spd in metabolism modulation. To sum up, this study adds to the
mounting evidence delineating the beneficial effects of Spd under specific adverse conditions. We show for the first time that Spd confers autophagy-dependent resistance to the neurotoxic
agent paraquat by improving survival and locomotor activity in _Drosophila_. Our work suggests that Spd might counteract neuronal damage caused by particular types of oxidative stress.
Future work, however, will have to test this speculation and address the mechanism(s) underlying the herein presented effects. On the other hand, we show that Spd is able to protect against
a different type of oxidative stress (H2O2) but in an autophagy-independent manner. Thus, Spd is able to promote survival with or without the involvement of autophagy, depending on the
specific oxidative stress scenario. MATERIALS AND METHODS FLIES AND REAGENTS Flies from an isogenized w1118 strain were used in all the experiments. The lines for the generation of
_Atg7__–/–_ flies were kindly provided by T Neufeld (University of Minnesota, Minneapolis, MN, USA).31 The homozygote mutants, _Atg7__d14__/Atg7__d77_, are homozygous mutants for _Atg7_.
Flies were kept in 25 °C, 70% humidity, 12 h light/12 h dark incubator, on an agar-cornmeal-sugar-yeast standard diet. Spermidine (Sigma, St. Louis, MO, USA, Ref S4139) was prepared as a 1-M
stock solution in sterile distilled water, aliquoted in single-use portions and stored at –20°C. New stock solution was prepared once a month. Paraquat (Sigma, Ref 856177) was prepared as a
1-M stock solution in sterile distilled water and stored at 4 °C. H2O2 (Sigma, Ref H3410) was purchased as a 30% solution and kept at 4°C. It was diluted as specified just before use.
PARAQUAT RESISTANCE Around 20 5-day-old flies were put in each vial containing filter papers (Sigma, Ref Z274852) soaked with 1.5 ml of a solution consisting of 5% glucose, 20 mM paraquat
and either 0, 0.01, 0.1 or 1 mM Spd, with an average of 87 flies in each group. Both w1118 and atg7−/− flies were studied, yielding a total of 3622 and 3427 observed flies, respectively.
Paraquat resistance was measured in both males and females, which were kept in separate vials. Solutions were renewed every other day until the last fly died. Vials were checked for dead
flies every 24 h. Comparison of survivorship for pooled data of all replicates was performed using log rank or Wilcoxon survival tests and corrected for multiple comparisons against the
control group. Each genotype and sex was analyzed separately. LOCOMOTOR ACTIVITY Ten 5-day-old flies were put in a vial containing filter papers soaked with 1.5 ml of a solution consisting
of 5% glucose, 5 mM paraquat and with or without 0.1 mM Spd with an average of 99 flies in each group. Both w1118 and atg7−/− flies were studied, yielding a total of 1179 and 1188 observed
flies, respectively. Locomotor activity was measured in both males and females, which were kept in separate vials. Vials were checked for dead flies every 24 h until the last fly died and
filters renewed every other day. Locomotor activity was measured daily until no fly could perform the task. Flies were moved to the bottom of their vial by mechanical stimulation and the
number of flies reaching the top of the vial (8 cm) in 10 s was recorded. The difference with Spd on the proportion of flies able to perform the climbing task was tested for each sex,
genotype and age (hours after beginning of exposure to paraquat) separately using non-parametric _z_ tests for proportions. Comparison of survivorship of pooled data was performed using
log-rank survival tests. Each genotype and sex was analyzed separately. HYDROGEN PEROXIDE RESISTANCE Around 20 5-day-old flies were put in a vial in containing filter papers soaked with 1.5
ml of a solution consisting of 5% glucose and 1 or 2% H2O2 without or with either 0.01, 0.1 or 1 mM Spd, with an average of 97 flies in each group for 1% H2O2 and 98 flies in each group for
2%. Both w1118 and atg7−/− flies were studied, yielding a total of 2329 and 2353 observed flies, respectively, for 1% H2O2, and 2360 and 2356 observed flies, respectively, for 2% H2O2. H2O2
resistance was measured in both males and females, which were kept in separate vials. Solutions were renewed every other day until the last fly died. Vials were checked for dead flies every
24 h. Comparison of survivorship data of pooled data was performed using log rank or Wilcoxon survival tests. Each genotype and sex was analyzed separately. STARVATION RESISTANCE Around 20
5-day-old flies were put in a vial containing only water and agar without or with either 0.01, 0.1 or 1 mM Spd, with an average of 99 flies in each group. Only w1118 flies were studied,
yielding a total of 2382 observed flies. Starvation resistance was measured in both males and females, which were kept in separate vials. Vials were renewed every other day until the last
fly died. Vials were checked for dead flies every 24 h. Comparison of survivorship of pooled data was performed using log rank or Wilcoxon survival tests. Each sex was analyzed separately.
ABBREVIATIONS * ATG7: autophagy-related gene 7 * Spd: spermidine REFERENCES * Vaupel JW . Biodemography of human ageing. _Nature_ 2010; 464: 536–542. Article CAS PubMed PubMed Central
Google Scholar * Kenyon CJ . The genetics of ageing. _Nature_ 2010; 464: 504–512. Article CAS PubMed Google Scholar * Fontana L, Partridge L, Longo VD . Extending healthy life span—from
yeast to humans. _Science_ 2010; 328: 321–326. CAS PubMed PubMed Central Google Scholar * Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A _et al_. Resveratrol improves
health and survival of mice on a high-calorie diet. _Nature_ 2006; 444: 337–342. Article CAS PubMed PubMed Central Google Scholar * Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR,
de Cabo R _et al_. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. _J Gerontol_ 2011; 66: 191–201. Article Google Scholar * Harrison DE,
Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K _et al_. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. _Nature_ 2009; 460: 392–395. Article CAS PubMed
PubMed Central Google Scholar * Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A _et al_. Mechanisms of life span extension by rapamycin in the fruit fly _Drosophila
melanogaster_. _Cell Metab_ 2010; 11: 35–46. Article CAS PubMed PubMed Central Google Scholar * Harrison B, Tran TT, Taylor D, Lee SD, Min KJ . Effect of rapamycin on lifespan in
_Drosophila_. _Geriatr Gerontol Int_ 2010; 10: 110–112. Article PubMed Google Scholar * Nishimura K, Shiina R, Kashiwagi K, Igarashi K . Decrease in polyamines with aging and their
ingestion from food and drink. _J Biochem_ 2006; 139: 81–90. Article CAS PubMed Google Scholar * Carmona-Gutiérrez D, Bauer MA, Ring J, Knauer H, Eisenberg T, Büttner S _et al_. The
propeptide of yeast cathepsin D inhinbits programmed necrosis. _Cell Death Dis_ 2011; 19: e161. Article Google Scholar * Eisenberg T, Knauer H, Schauer A, Fussi H, Büttner S,
Carmona-Gutierrez D _et al_. Induction of autophagy by spermidine promotes longevity. _Nat Cell Biol_ 2009; 11: 1305–1314. Article CAS PubMed Google Scholar * Suppola S, Heikkinen S,
Parkkinen JJ, Uusi-Oukari M, Korhonen VP, Keinänen T _et al_. Concurrent overexpression of ornithine decarboxylase and spermidine/spermine N1-acetyltransferase further accelerates the
catabolism of hepatic polyamines in transgenic mice. _Biochem J_ 2001; 358: 343–348. Article CAS PubMed PubMed Central Google Scholar * Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F
. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. _Exp Gerontol_ 2009; 44: 727–732. Article CAS PubMed Google Scholar * Kaeberlein M . Spermidine
surprise for a long life. _Nat Cell Biol_ 2009; 11: 1277–1278. Article CAS PubMed Google Scholar * Gonidakis S, Finkel SE, Longo VD . Lifespan extension and paraquat resistance in a
ubiquinone-deficient _Escherichia coli_ mutant depend on transcription factors ArcA and TdcA. _Aging_ 2011; 3: 291–303. Article CAS PubMed PubMed Central Google Scholar * Fabrizio P,
Pozza F, Pletcher SD, Gendron CM, Longo VD . Regulation of longevity and stress resistance by Sch9 in yeast. _Science_ 2001; 292: 288–290. Article CAS PubMed Google Scholar * Bagatini
PB, Saur L, Rodrigues MF, Bernardino GC, Paim MF, Coelho GP _et al_. The role of calcium channel blockers and resveratrol in the prevention of paraquat-induced parkisonism in _Drosophila
melanogaster_: a locomotor analysis. _Invert Neurosci_ 2011; 11: 43–51. Article CAS PubMed Google Scholar * Vellai T . Autophagy genes and ageing. _Cell Death Differ_ 2009; 16: 94–102.
Article CAS PubMed Google Scholar * Morselli E, Mariño G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S _et al_. Spermidine and resveratrol induce autophagy by distinct pathways
converging on the acetylproteome. _J Cell Biol_ 2011; 192: 615–629. Article CAS PubMed PubMed Central Google Scholar * Madeo F, Eisenberg T, Büttner S, Ruckenstuhl C, Kroemer G .
Spermidine: a novel autophagy inducer and longevity elixir. _Autophagy_ 2010; 6: 160–162. Article PubMed Google Scholar * Minois N, Carmona-Gutierrez D, Madeo F . Polyamines in aging and
disease. _Aging_ 2011; 3: 716–732. Article PubMed PubMed Central Google Scholar * Lithgow GJ, White TM, Melov S, Johnson TE . Thermotolerance and extended life-span conferred by
single-gene mutations and induced by thermal stress. _Proc Natl Acad Sci USA_ 1995; 92: 7540–7544. Article CAS PubMed PubMed Central Google Scholar * Lin YR, Kim K, Yang Y, Ivessa A,
Sadoshima J, Park Y . Regulation of longevity by regulator of G-protein signaling protein, Loco. _Aging Cell_ 2011; 10: 438–447. Article CAS PubMed Google Scholar * Lee GD, Wilson MA,
Zhu M, Wolkow CA, de Cabo R, Ingram DK _et al_. Dietary deprivation extends lifespan in _Caenorhabditis elegans_. _Aging Cell_ 2006; 5: 515–524. Article CAS PubMed Google Scholar *
Reverter-Branchat G, Cabiscol E, Tamarit J, Ros J . Oxidative damage to specific proteins in replicative and chronological-aged _Saccharomyces cerevisiae_: common targets and prevention by
calorie restriction. _J Biol Chem_ 2004; 279: 31983–31989. Article CAS PubMed Google Scholar * Burger JM, Hwangbo DS, Corby-Harris V, Promislow DE . The functional costs and benefits of
dietary restriction in _Drosophila_. _Aging Cell_ 2007; 6: 63–71. Article CAS PubMed Google Scholar * Jimenez-Del-Rio M, Guzman-Martinez C, Velez-Pardo C . The effects of polyphenols on
survival and locomotor activity in _Drosophila melanogaster_ exposed to iron and paraquat. _Neurochem Res_ 2010; 35: 227–238. Article CAS PubMed Google Scholar * Xiong Y, Contento AL,
Nguyen PQ, Bassham DC . Degradation of oxidized proteins by autophagy during oxidative stress in _Arabidopsis_. _Plant Physiol_ 2007; 143: 291–299. Article CAS PubMed PubMed Central
Google Scholar * González-Polo RA, Niso-Santano M, Ortíz-Ortíz MA, Gómez-Martín A, Morán JM, García-Rubio L _et al_. Inhibition of paraquat-induced autophagy accelerates the apoptotic cell
death in Neuroblastoma SH-SY5Y cells. _Toxicol Sci_ 2007; 97: 448–458. Article PubMed Google Scholar * Girardot F, Monnier V, Tricoire H . Genome wide analysis of common and specific
stress responses in adult _Drosophila melanogaster_. _BMC Genomics_ 2004; 5: 74. Article PubMed PubMed Central Google Scholar * Juhász G, Èrdi B, Sass M, Neufeld TP . Atg7-dependent
autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in _Drosophila_. _Genes Dev_ 2007; 21: 3061–3066. Article PubMed PubMed Central
Google Scholar * Poon PC, Kuo TH, Linford NJ, Roman G, Pletcher SD . Carbon dioxide sensing modulates lifespan and physiology in _Drosophila_. _PLoS Biol_ 2010; 8: e1000356. Article PubMed
PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the University of St. Andrews, as well as by the Austrian Science Fund FWF (grants LIPOTOX
and P23490-B12 to FM; P24381-B20 to FM and TE) and the European Commission (Apo-Sys to FM and TE). Furthermore, this work was supported by grants to GK from the Ligue Nationale contre le
Cancer (Equipe labellisée), Agence Nationale pour la Recherche (ANR), Cancéropôle Ile-de-France, European Commission (Apo-Sys, ArtForce), Fondation Axa (Chair for longevity research),
Fondation pour la Recherche Médicale (FRM), Institut National du Cancer (INCa), et Fondation Bettencourt-Schueller. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Biology,
Biomedical Sciences Research Complex, University of St. Andrews, St. Andrews, UK N Minois * Institute for Molecular Biosciences, University of Graz, Graz, Austria D Carmona-Gutierrez, M A
Bauer, P Rockenfeller, T Eisenberg & F Madeo * Department of Biological Sciences and Norris Cancer Center, Andrus Gerontology Center, University of Southern California, Los Angeles, CA,
USA S Brandhorst * Centre for Medical Biotechnology, Faculty of Biology, University Duisburg–Essen, Essen, Germany S Brandhorst * Department of Genetics, Institute for Biology, Free
University Berlin, Berlin, Germany S J Sigrist * Neurocure Cluster of Excellence, Charité Berlin, Berlin, Germany S J Sigrist * INSERM, U848, Institute Gustave Roussy, University Paris XI,
Villejuif, France G Kroemer * Metabolomics Platform, Institut Gustave Roussy, Villejuif, France G Kroemer * Centre de Recherche des Cordeliers, Université Paris Descartes, Paris 5, Paris,
France G Kroemer * Pôle de Biologie, Hôpital Européen Georges Pompidou, AP-HP, Paris, France G Kroemer Authors * N Minois View author publications You can also search for this author
inPubMed Google Scholar * D Carmona-Gutierrez View author publications You can also search for this author inPubMed Google Scholar * M A Bauer View author publications You can also search
for this author inPubMed Google Scholar * P Rockenfeller View author publications You can also search for this author inPubMed Google Scholar * T Eisenberg View author publications You can
also search for this author inPubMed Google Scholar * S Brandhorst View author publications You can also search for this author inPubMed Google Scholar * S J Sigrist View author publications
You can also search for this author inPubMed Google Scholar * G Kroemer View author publications You can also search for this author inPubMed Google Scholar * F Madeo View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to N Minois or D Carmona-Gutierrez. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no conflict of interest. ADDITIONAL INFORMATION Edited by M Piacentini Supplementary Information accompanies the paper on Cell Death and Disease website SUPPLEMENTARY
INFORMATION SUPPLEMENTARY MATERIAL (DOC 683 KB) RIGHTS AND PERMISSIONS This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a
copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Minois, N., Carmona-Gutierrez, D., Bauer, M. _et
al._ Spermidine promotes stress resistance in _Drosophila melanogaster_ through autophagy-dependent and -independent pathways. _Cell Death Dis_ 3, e401 (2012).
https://doi.org/10.1038/cddis.2012.139 Download citation * Received: 27 March 2012 * Revised: 31 July 2012 * Accepted: 03 September 2012 * Published: 11 October 2012 * Issue Date: October
2012 * DOI: https://doi.org/10.1038/cddis.2012.139 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link
is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * _Drosophila melanogaster_ * spermidine *
paraquat * oxidative stress * activity * starvation