Novel roles for herg k+ channels in cell proliferation and apoptosis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The human ether-a-go-go-related gene potassium channel (hERG, Kv11.1, KCNH2) has an essential role in cardiac action potential repolarization. Electrical dysfunction of the
voltage-sensitive ion channel is associated with potentially lethal ventricular arrhythmias in humans. hERG K+ channels are also expressed in a variety of cancer cells where they control
cell proliferation and apoptosis. In this review, we discuss molecular mechanisms of hERG-associated cell cycle regulation and cell death. In addition, the significance of hERG K+ channels
as future drug target in anticancer therapy is highlighted. SIMILAR CONTENT BEING VIEWED BY OTHERS MAPPING THE FUNCTIONAL EXPRESSION OF AUXILIARY SUBUNITS OF KCA1.1 IN GLIOBLASTOMA Article
Open access 20 December 2022 ROLE OF TRPM2 IN BRAIN TUMOURS AND POTENTIAL AS A DRUG TARGET Article 09 June 2021 KV1.3 VOLTAGE-GATED POTASSIUM CHANNELS LINK CELLULAR RESPIRATION TO
PROLIFERATION THROUGH A NON-CONDUCTING MECHANISM Article Open access 07 April 2021 ION CHANNELS INVOLVED IN CELL PROLIFERATION AND DEATH Ion channels have been implicated in signaling
pathways leading to cell proliferation or apoptosis (programmed cell death). Their identification and functional characterization in tumor cells suggest potential significance in anticancer
therapy. Transient receptor potential channels form a superfamily of ubiquitously expressed channels influencing the balance between cell survival and death.1, 2 In addition,
hyperpolarization-activated cyclic nucleotide-gated channels were detected in embryonic stem cells where they exert pro-proliferatory effects. Potassium channels represent the largest group
of channels involved in cell death and proliferation.3, 4 Calcium-activated KCa3.1 channels contribute to proliferation and atherosclerosis, and inhibition of the current attenuates fibrosis
and lymphocyte proliferation.5, 6, 7, 8 Furthermore, voltage-gated K+ channels (e.g. Kv1.3) or two-pore-domain channels (e.g. K2P5.1) determine growth of adenocarcinomas.9, 10
Voltage-sensitive human ether-a-go-go-related gene (hERG) potassium channels have recently emerged as novel regulators of growth and death in cancer cells. This review focuses on hERG
channels in proliferation and apoptosis. Current knowledge on expression, function and regulation is reviewed, and clinical implications are discussed. DIFFERENTIAL EXPRESSION OF HERG
POTASSIUM CHANNELS CARDIAC EXPRESSION AND FUNCTION OF HERG K+ CHANNELS. Repolarization of cardiac ventricular myocytes is mainly regulated by outward potassium currents. One of the most
important currents is the delayed rectifier potassium current, _I_K, which has rapidly and slowly activating components (_I_Kr and _I_Ks).11 Activation of the rapid component of the delayed
rectifier potassium current, _I_Kr, terminates the plateau phase and initiates repolarization of the cardiac action potential. The hERG encodes the voltage-gated potassium channel
_α_-subunit underlying _I_Kr.12, 13, 14 hERG potassium channels form homo-tetramers of identical six transmembrane spanning domains, with a cluster of positive charges localized in the S4
domain serving as voltage sensor. hERG channels are a primary target for the pharmacological management of arrhythmias with class III antiarrhythmic agents.15, 16 Blockade of hERG currents
causes lengthening of the cardiac action potential, which may produce a beneficial class III antiarrhythmic effect. Excessive reduction of HERG currents due to mutations in hERG or _via_
blockade produces chromosome-7-linked congenital long QT syndrome (LQTS-2) and acquired long QT syndrome, respectively. Both forms of LQTS are associated with delayed cardiac repolarization,
prolonged electrocardiographic QT intervals, and a risk for the development of ventricular ‘torsade de pointes’ arrhythmias and sudden cardiac death. hERG channels are inhibited by a
variety of non-antiarrhythmic compounds. This undesirable side effect is now considered a significant hurdle in the development of new and safer drugs, and has forced removal of several
drugs from the market. In addition to LQTS, cardiomyocyte apoptosis has been reported following pharmacological hERG K+ channel blockade.17 HERG K+ CHANNELS IN CANCER Various cancer cell
lines of epithelial, neuronal, leukemic, and connective tissue origin express hERG K+ channels (Table 1), whereas corresponding non-cancerous cells and cell lines do not exhibit significant
hERG protein levels. In corresponding human cancers, hERG protein may serve as biomarkers of malignant transition. Furthermore, hERG expression is implicated in enhanced cell proliferation,
invasiveness, lymph node dissemination, and reduced cell differentiation and prognosis.21, 22 In addition, increased neoangiogenesis, another hallmark of malignant tissue growth, has been
reported for glioblastoma where the generation of blood vessels was stimulated by hERG-dependent secretion of vascular endothelial growth factor.27 DIFFERENTIAL HERG EXPRESSION PATTERNS
DURING ONTOGENESIS. While hERG expression in normal adult human tissue is limited to heart, brain, myometrium, pancreas, and hematopoietic progenitors, other species have been described to
undergo changes in their ERG expression profile during ontogenesis: quail embryos express ERG K+ channels in peripheral ganglia and skeletal muscle in addition to heart and central nervous
system.47 This observation illustrates that hERG expression in tumor cells might either represent ectopic re-expression of a gene that remains silent in differentiated cells, or reflect
re-activation of embryonic genes, which is well recognized in cancers.35 CELL PROLIFERATION FUNCTIONAL ROLE OF HERG K+ CHANNELS IN CELL PROLIFERATION In differentiated adult cells, resting
membrane potential varies from −40 mV to about −90 mV.48 These distinct differences are closely correlated to the proliferative potential of respective cell types, ranging from slowly
proliferating or non-proliferative neurons or muscle cells (−70 mV to −90 mV) to highly proliferative glandular epithelia of liver, thyroid, pancreas, or salivary glands (−40 mV to −55
mV).48 hERG K+ channels are closed at membrane potentials below a threshold of ∼−60 mV1 whereas classical inwardly rectifying channels remain open at more negative membrane potentials.49 The
predominance of hERG in cycling cells may thus account for the depolarized resting membrane potential in these cells.31 The membrane potential of cycling cells is particularly depolarized
during the G1 phase. However, K+ channel-dependent hyperpolarization appears to be critical for progression to the S phase. Hyperpolarization evokes Ca2+ influx, which is further augmented
by calcium-dependent K+ (KCa) channels and permits synthesis of mitogenic factors. In addition, hyperpolarization provides the electrical gradient necessary for Na+-dependent transport of
metabolic substrates and ions across the plasma membrane, which is required for DNA synthesis.50 Considering that K+ channels are involved in cell cycle progression, abundant expression of
K+ channels is expected to cause loss of proliferative control if endogenous pathways fail to block excessively expressed K+ channels.50 Interestingly, the promoter region of the hERG gene
harbors multiple binding sites for oncoproteins, such as specificity protein 1 and nuclear factor kappa light chain enhancer of activated B-cells, and for the tumor suppressor protein Nkx3.1
(Nk3 homeobox 1).30 We may hypothesize that mutations in oncoproteins constitutively activate hERG gene expression, shifting resting membrane potentials of cancerous cells toward more
depolarized values and repolarizing them at the end of G1 phase, thereby facilitating cell cycle progression and thus leading to cell proliferation. Here, pharmacological intervention using
hERG antagonists will serve to arrest the cell cycle in the G1 phase. Furthermore, human gastric cancer cells exhibit reduced levels of the regulatory _β_-subunit KCNE2, leading to hERG
current increase.51, 52 In addition, genetic deletion of KCNE2 is associated with gastric neoplasia and increased nuclear cyclin D1 levels in mice, revealing genetic manipulation of cell
proliferation mediated by a hERG _β_-subunit.52 Various cancer cell lines and cardiomyocytes have been reported to express an N terminally truncated splice variant of hERG, hERG1b, that
confers specific electrophysiological properties.53 Pharmacological approaches targeting the hERG1/hERG1b ratio may modulate the resting membrane potential of cycling cells. Increased hERG1b
levels are expected to depolarize cells, while high hERG1 levels will shift membrane potential toward more hyperpolarized values35 and suppress cell proliferation. HERG POTASSIUM CHANNEL
BLOCKERS MODULATE PROLIFERATION. Leukemic cell lines express hERG K+ channels whereas non-cancerous lymphocytes do not exhibit hERG protein. Selective hERG channel blockade by E-4031 reduced
proliferation in cancerous cell lines.25 Unspecific deceleration of the cell cycle and reduction of cell proliferation50 were ruled out in mechanistic analyses, confirming specific cell
cycle arrest as underlying mechanism. Cell cycle analysis of FLG29.1 leukemia cells revealed accumulation of cells in the G1 phase following treatment with hERG channel blockers.24
Furthermore, additional structurally different hERG blockers have been shown to achieve cell cycle arrest in G1 phase of hERG-positive cells (Table 2). It is noteworthy that the hERG blocker
erythromycin blocks cell cycle in G2 phase if administered together with vincristine.29 In addition, hERG blockers doxazosin and terazosin did not cause cell cycle arrest despite hERG
expression in distinct cell lines, for example, LNCaP prostate carcinoma cells.30, 57 SIGNIFICANCE OF HERG ION CHANNELS IN APOPTOSIS PROAPOPTOTIC EFFECTS OF HERG K+ CHANNEL INHIBITORS. hERG
channel blockers have been shown to induce apoptosis in different cell types. This mechanism is independent of their capacity to inhibit cell proliferation via cell cycle arrest. The
significance of hERG K+ channels in apoptotic pathways has been demonstrated in hERG-transfected HEK293 cells, which underwent apoptosis upon administration of doxazosin, compared with
control HEK293 cells lacking endogenous hERG.58 Doxazosin is an _α_1-adrenocepor antagonist with hERG-blocking properties that is clinically used as antihypertensive drug.59 In the
Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), which compared novel antihypertensive drugs to diuretic treatment in 33 000 patients, the doxazosin arm
had to be discontinued due to an increase in congestive heart failure that may be attributed to cardiomyocyte apoptosis.60, 61 The proapoptotic effect of doxazosin has been confirmed _in
vitro_ in the murine atrial tumor cell line HL-1 and in isolated adult human cardiomyocytes,17 providing a possible explanation for the increased incidence of congestive heart failure in the
doxazosin arm of the ALLHAT trial. In addition to hypertension, doxazosin is used for treatment of lower urinary tract symptoms caused by benign prostatic hyperplasia (BPH). Smooth muscle
relaxation due to _α_1-adrenergic blockade was initially thought to underlie the relief of symptoms in BPH patients. However, subsequent studies revealed an apoptotic effect of doxazosin in
hyperplastic prostatic tissue that may contribute to its clinical efficacy.62 Furthermore, doxazosin induced apoptosis in prostatic cancer cells.63 Limitations arise from the lack of studies
directly comparing hERG expression in normal, hyperplastic, and cancerous prostatic tissue, respectively. Finally, hERG channel expression is well documented in pituitary adenoma cells.45
When treated with doxazosin _in vitro_, antiproliferative and proapoptotic effects were observed in pituitary adenoma cells independent of antiadrenergic properties of the drug.55 MOLECULAR
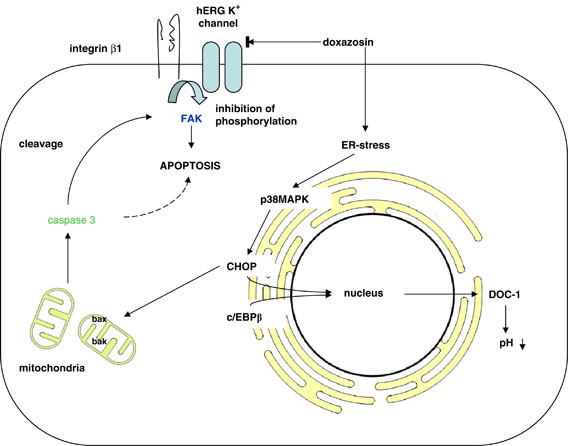
MECHANISMS OF HERG-ASSOCIATED APOPTOSIS. hERG K+ channel blockers such as doxazosin activate multiple apoptotic pathways. However, evidence for a direct mechanistic link between hERG K+
channels and apoptotic proteins remains sparse to date. In HL-1 cardiomyocytes, doxazosin induces apoptosis via the endoplasmic reticulum pathway, involving enhanced phosphorylation of p38
mitogen-activated protein kinase, which activates GADD153/CHOP (growth arrest and DNA damage-induced gene 153/c/EBP homologous protein). GADD153/CHOP subsequently forms heterodimers with
DNA-binding protein c/EBP_β_ (CCAAT enhancer-binding protein beta) and translocates into the nucleus, where it augments transcription of the carbonic anhydrase DOC-1 (downstream of CHOP-1).
DOC-1 then acidifies intracellular pH and facilitates apoptosis.64 Finally, the CHOP pathway results in activation of a key apoptotic enzyme, caspase 3.65 Caspase activation by doxazosin
induces cleavage of the protein-tyrosine kinase FAK (focal adhesion kinase) in HL-1 cells, which compromises cell adhesion and leads to apoptosis.64 FAK is an essential component of integrin
signaling and is phosphorylated when cells are adhered to the extracellular matrix. Thus, it provides a survival signal and prevents apoptosis.66 In prostate cancer cells, FAK is cleaved by
caspase 3 upon treatment with doxazosin, which leads to apoptosis or anoikis (i.e. apoptosis due to loss of cell adhesion).67 Furthermore, hERG1, integrin _β_1, and FAK form a
macromolecular complex in hERG1-transfected HEK293 cells and SH-SY5Y neuroblastoma cells. Cell adhesion via integrin _β_1 causes activation of hERG1, which is essential for direct FAK
phosphorylation (Figure 1).37 FAK and hERG overexpression have independently been related to enhanced dissemination and invasiveness of tumors.20, 66 FAK phosphorylation due to hERG
activation may explain the ability of malignant cells to circumvent apoptosis once they have lost contact to the extracellular matrix. The abundant expression of hERG and FAK might provide
crucial survival signals in the absence of cell adhesion, and thus account for increased invasiveness and dissemination of hERG-positive tumors. In addition, colocalization with hERG
potassium channels activates the GTPase Rac1 and may contribute to adhesion-dependent modulation of tumor cell motility.37 Cell type- and environment-specific effects on apoptosis are
suggested by reports of hERG activity promoting apoptosis. In hERG-positive SKBr3, SH-SY5Y, and HL-1 cells, apoptosis occurs via a caspase 3-dependent pathway in response to extracellular
administration of H2O2 or TNF_α_ (tumor necrosis factor _α_), whereas selective inhibition of hERG conductance by dofetilide attenuates the proapoptotic effect of H2O2 and TNF_α_.33 The
methodology in the latter study is different from investigations mentioned above. Cells were first incubated with H2O2 or TNF_α_ to induce apoptosis, followed by application of hERG
blockers. In the same study, hERG is revealed to recruit TNF_α_ receptor 1 to the plasma membrane, which might explain increased responsiveness to TNF_α_ in these cells.33 The authors
describe a proliferative effect in hERG-expressing cells at low doses of TNF_α_ and an antiapoptotic effect of the hERG inhibitor dofetilide upon pretreatment with H2O2 and TNF_α_. These
observations appear to be at odds with proapoptotic effects of hERG K+ channel blockers. The hERG blocker doxazosin has been proven as a proapoptotic agent in a wide range of _in vitro_ and
_in vivo_ studies. Doxazosin increases the intracellular H2O2 content in BPH stromal cells. This is considered to facilitate TNF_α_-related pathways.68 Administration of H2O2 before hERG
inhibition appears to interfere with hERG-induced signaling pathways, which augment intracellular H2O2 levels. The antiapoptotic effect of hERG channel blockade may be due to this
interference. However, pro- and antiapoptotic effects of hERG blockers might coexist, and proapoptotic effects, including the increase in intracellular H2O2, could outweigh a possible
antiapoptotic effect through suppression of the apoptotic H2O2 – TNF_α_ pathway. However, an unambiguous differentiation between effects of hERG conductance and hERG expression is lacking,
and the mechanism by which hERG conductance facilitates H2O2- and TNF_α_-mediated apoptosis remains unclear at the molecular level. CLINICAL AND THERAPEUTIC IMPLICATIONS DIAGNOSTIC VALUE OF
HERG K+ CHANNEL EXPRESSION IN TUMORS hERG may be utilized as a potential tumor marker, given their expression in a variety of tumor cells and their absence from most non-cancerous human
tissues. Specifically, hERG was detected in endometrial cancer at mRNA (sensitivity=67%; _n_=18) and protein levels (sensitivity=82%; _n_=18), whereas only 18% (_n_=11) of non-cancerous
endometrial samples exhibited hERG mRNA or protein.23 In colon carcinomas, hERG mRNA was a more sensitive and more specific indicator for malignancy (100% sensitivity and specificity;
_n_=23) than mRNA of the established tumor markers CEA (sensitivity=94.4%; _n_=18), CK19 (sensitivity=77.8%; _n_=18), or CK20 (sensitivity=94.4%; _n_=18).18 Immunohistochemical staining for
hERG protein reached similar sensitivity and specificity as hERG mRNA.18 Further validation is required in larger patient populations. PROGNOSTIC SIGNIFICANCE OF HERG K+ CHANNEL EXPRESSION
IN TUMORS The prognostic value of hERG expression in tumors has been evaluated in several tissues. In acute myeloid leukemia (AML) blasts, hERG K+ channel expression is associated with a 50%
reduction of relapse-free and overall survival time compared with patients with hERG-negative AML (12 _versus_ 23 months).69 Patients with esophageal squamous cell carcinomas similarly
exhibit reduced survival (30 _versus_ 56 months) when hERG is detected.22 However, hERG K+ channel expression was not significantly associated with invasiveness, dissemination, or tumor
grade in this study. In gastric cancer cells, levels of hERG expression are positively correlated to tumor de-differentiation and TNM stage.21 Moreover, tumor growth was observed in BALB/c
nu/nu mice following injection of gastric cancer cells. Injection of cancer cells that were pretreated with hERG siRNA significantly attenuated tumorigenesis,21 confirming the pathological
significance of hERG in tumor growth and suggesting a potential novel target in anticancer therapy (see below). In colonic adenocarcinomas, there is a significant correlation between hERG K+
channel expression and invasiveness or dissemination. hERG is not detected in normal colonic mucosa (0%; _n_=60) and rarely observed in adenoma (9%; _n_=11). In contrast, substantial hERG
was found in patients with non-metastatic adenocarcinoma (75%; _n_=52) and metastatic adenocarcinoma (100%; _n_=8), with the most pronounced staining found in hepatic and peritoneal
metastasis.20 ANTICANCER THERAPY The antihypertensive _α_1-adrenoceptor blocker doxazosin is an established treatment option in BPH. Its therapeutic efficacy has been attributed to induction
of apoptosis in hyperplastic and cancerous prostate cells.57 Furthermore, hERG-positive cancer cells have been reported to be particularly susceptible to chemotherapeutics vincristine,
paclitaxel, and hydroxycamptothecin.29 Direct effects of vincristine, paclitaxel, and hydroxycamptothecin on hERG channels remain to be investigated. Erythromycin, a macrolide antibiotic
with hERG-blocking properties, further enhances the antiproliferative effect of these chemotherapeutics.29 The most intriguing perspective of anticancer therapy targeting hERG channels is
direct blockade of the potassium channel, which is expected to produce antiproliferative and proapoptotic effects that diminish tumor growth and invasiveness. The first proof of concept
study confirmed prevention of gastric cancer cell proliferation by the hERG K+ channel blocker cisapride.70 A systematic _in vivo_ investigation of chemotherapeutic properties and potential
cardiac side effects of hERG inhibitors is required. POTENTIAL SIDE EFFECTS AND LIMITATIONS OF ANTICANCER THERAPY BASED ON HERG CURRENT INHIBITION Proarrhythmic14 and cardiotoxic risks of
hERG inhibitors require careful evaluation7 when applying these compounds in clincial oncology. Systemic treatment of cancers with hERG antagonists may affect cardiac myocytes, resulting in
apoptosis and heart failure. In addition, application of hERG antagonists may induce QT prolongation and ventricular tachycardia. Although cancer treatment usually occurs in life-threatening
situations, and in some cases potential cardiac damage is accepted (e.g. during use of anthracyclines), optimal suppression of these events will be required. To prevent proarrhythmic side
effects, short-term drug application may be sufficient to induce apoptosis in tumor cells with minimal effects on cardiac electrophysiology. ECG monitoring should be performed during
application of the drug. Additional pharmacological inhibition of cardiac L-type calcium channels or _β_-adrenoceptors may offset the limiting proarrhythmic effects of hERG channel
inhibitors.71, 72, 73 Cardiomyocyte apoptosis may be circumvented through targeted delivery techniques such as direct injection or trans-arterial drug application. Gene therapy represents an
additional therapeutic approach to targeted suppression of hERG channel expression in cancers. Different proliferative states of cardiac and tumor cells may render cancerous tissue more
susceptible to pro-apoptotic and antiproliferative stimuli, reducing the overall risk of heart failure during systemic application of hERG antagonists. Feasibility of tumor-selective
hERG-based anticancer therapy will further depend on differential drug effects on cancerous and non-cancerous tissue expressing hERG K+ channels. CONCLUSION hERG potassium channels,
previously recognized to promote cardiac action potential repolarization, are now revealed to serve as regulators of proliferation and apoptosis in cancer cells. Their significance in
anticancer therapy is supported by mechanistic data and preliminary _in vivo_ studies. Limitations arise from potential cardiac side effects that require attention. Further studies are
warranted to provide a more complete understanding of hERG effects on apoptotic pathways. Downstream signaling proteins may serve as more specific therapeutic drug targets in future
anticancer therapy. ABBREVIATIONS * ALLHAT: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial * AML: acute myeloid leukemia * BPH: benign prostatic hyperplasia *
CHOP: c/EBP homologous protein * DOC-1: downstream of CHOP-1 * FAK, focal adhesion kinase∣hERG: human ether-a-go-go-related gene * Kv: voltage-gated potassium channel * LQTS: long QT
syndrome * TNF_α_: tumor necrosis factor _α_ REFERENCES * Shapovalov G, Lehen’kyi V, Skryma R, Prevarskaya N . TRP channels in cell survival and cell death in normal and transformed cells.
_Cell Calcium_ 2011; e-pub ahead of print 29 May 2011; doi:10.1016/j.ceca.2011.05.006. * Lau YT, Wong CK, Luo J, Leung LH, Tsang PF, Bian ZX _et al_. Effects of hyperpolarization-activated
cyclic nucleotide-gated (HCN) channel blockers on the proliferation and cell cycle progression of embryonic stem cells. _Pflugers Arch_ 2011; 461: 191–202. Article CAS PubMed Google
Scholar * Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M . Potassium channels: molecular defects, diseases, and therapeutic opportunities. _Pharmacol Rev_ 2000; 52: 557–594. CAS PubMed
Google Scholar * Lang F, Föller M, Lang KS, Lang PA, Ritter M, Gulbins E _et al_. Ion channels in cell proliferation and apoptotic cell death. _J Membr Biol_ 2005; 205: 147–157. Article
CAS PubMed Google Scholar * Chou CC, Lunn CA, Murgolo NJ . KCa3.1: target and marker for cancer, autoimmune disorder and vascular inflammation? _Expert Rev Mol Diagn_ 2008; 8: 179–187.
Article CAS PubMed Google Scholar * Srivastava S, Zhdanova O, Di L, Li Z, Albaqumi M, Wulff H _et al_. Protein histidine phosphatase 1 negatively regulates CD4 T cells by inhibiting the
K+ channel KCa3.1. _Proc Natl Acad Sci USA_ 2008; 105: 14442–14446. Article CAS PubMed PubMed Central Google Scholar * Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T _et al_.
The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. _J Clin Invest_ 2008; 118: 3025–3037. Article CAS PubMed PubMed
Central Google Scholar * Grgic I, Kiss E, Kaistha BP, Busch C, Kloss M, Sautter J _et al_. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. _Proc Natl Acad
Sci USA_ 2009; 106: 14518–14523. Article CAS PubMed PubMed Central Google Scholar * Jang SH, Choi SY, Ryu PD, Lee SY . Anti-proliferative effect of Kv1.3 blockers in A549 human lung
adenocarcinoma _in vitro_ and _in vivo_. _Eur J Pharmacol_ 2011; 651: 26–32. Article CAS PubMed Google Scholar * Alvarez-Baron CP, Jonsson P, Thomas C, Dryer SE, Williams C . The
two-pore domain potassium channel KCNK5: induction by estrogen receptor {alpha} and role in proliferation of breast cancer cells. _Mol Endocrinol_ 2011; 25: 1326–1336. Article CAS PubMed
PubMed Central Google Scholar * Sanguinetti MC, Jurkiewicz NK . Two components of delayed rectifier K+ current. _J Gen Physiol_ 1990; 96: 195–215. Article CAS PubMed Google Scholar *
Warmke JW, Ganetzky B . A family of potassium channel genes related to eag in Drosophila and mammals. _Proc Natl Acad Sci USA_ 1994; 91: 3438–3442. Article CAS PubMed PubMed Central
Google Scholar * Sanguinetti MC, Jiang C, Curran ME, Keating MT . A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. _Cell_
1995; 81: 299–307. Article CAS PubMed Google Scholar * Sanguinetti MC, Tristani-Firouzi M . hERG potassium channels and cardiac arrhythmia. _Nature_ 2006; 440: 463–469. Article CAS
PubMed Google Scholar * Thomas D, Karle CA, Kiehn J . The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications. _Curr
Pharm Des_ 2006; 12: 2271–2283. Article CAS PubMed Google Scholar * Staudacher I, Schweizer PA, Katus HA, Thomas D . hERG: protein trafficking and potential for therapy and drug side
effects. _Curr Opin Drug Discov Devel_ 2010; 13: 23–30. CAS PubMed Google Scholar * Gonzalez-Juanatey JR, Iglesias MJ, Alcaide C, Pineiro R, Lago F . Doxazosin induces apoptosis in
cardiomyocytes cultured _in vitro_ by a mechanism that is independent of alpha1-adrenergic blockade. _Circulation_ 2003; 107: 127–131. Article CAS PubMed Google Scholar * Dolderer JH,
Schuldes H, Bockhorn H, Altmannsberger M, Lambers C, von Zabern D _et al_. HERG1 gene expression as a specific tumor marker in colorectal tissues. _Eur J Surg Oncol_ 2010; 36: 72–77. Article
CAS PubMed Google Scholar * Gong JH, Liu XJ, Shang BY, Chen SZ, Zhen YS . HERG K+ channel related chemosensitivity to sparfloxacin in colon cancer cells. _Oncol Rep_ 2010; 23:
1747–1756. CAS PubMed Google Scholar * Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, Witchel H _et al_. herg1 gene and HERG1 protein are overexpressed in colorectal cancers
and regulate cell invasion of tumor cells. _Cancer Res_ 2004; 64: 606–611. Article CAS PubMed Google Scholar * Shao XD, Wu KC, Guo XZ, Xie MJ, Zhang J, Fan DM . Expression and
significance of HERG protein in gastric cancer. _Cancer Biol Ther_ 2008; 7: 45–50. Article CAS PubMed Google Scholar * Ding XW, Luo HS, Luo B, Xu DQ, Gao S . Overexpression of hERG1 in
resected esophageal squamous cell carcinomas: a marker for poor prognosis. _J Surg Oncol_ 2008; 97: 57–62. Article CAS PubMed Google Scholar * Cherubini A, Taddei GL, Crociani O,
Paglierani M, Buccoliero AM, Fontana L _et al_. HERG potassium channels are more frequently expressed in human endometrial cancer as compared to non-cancerous endometrium. _Br J Cancer_
2000; 83: 1722–1729. Article CAS PubMed PubMed Central Google Scholar * Pillozzi S, Brizzi MF, Balzi M, Crociani O, Cherubini A, Guasti L _et al_. HERG potassium channels are
constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. _Leukemia_ 2002; 16: 1791–1798. Article CAS
PubMed Google Scholar * Smith GA, Tsui HW, Newell EW, Jiang X, Zhu XP, Tsui FW _et al_. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. _J Biol Chem_
2002; 277: 18528–18534. Article CAS PubMed Google Scholar * Li H, Liu L, Guo L, Zhang J, Du W, Li X _et al_. HERG K+ channel expression in CD34+/CD38−/CD123(high) cells and primary
leukemia cells and analysis of its regulation in leukemia cells. _Int J Hematol_ 2008; 87: 387–392. Article CAS PubMed Google Scholar * Masi A, Becchetti A, Restano-Cassulini R, Polvani
S, Hofmann G, Buccoliero AM _et al_. hERG1 channels are overexpressed in glioblastoma multiforme and modulate VEGF secretion in glioblastoma cell lines. _Br J Cancer_ 2005; 93: 781–792.
Article CAS PubMed PubMed Central Google Scholar * Patt S, Preussat K, Beetz C, Kraft R, Schrey M, Kalff R _et al_. Expression of ether a go-go potassium channels in human gliomas.
_Neurosci Lett_ 2004; 368: 249–253. Article CAS PubMed Google Scholar * Chen SZ, Jiang M, Zhen YS . HERG K+ channel expression-related chemosensitivity in cancer cells and its modulation
by erythromycin. _Cancer Chemother Pharmacol_ 2005; 56: 212–220. Article CAS PubMed Google Scholar * Lin H, Xiao J, Luo X, Wang H, Gao H, Yang B _et al_. Overexpression HERG K(+)
channel gene mediates cell-growth signals on activation of oncoproteins SP1 and NF-kappaB and inactivation of tumor suppressor Nkx3.1. _J Cell Physiol_ 2007; 212: 137–147. Article CAS
PubMed Google Scholar * Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P _et al_. herg encodes a K+ current highly conserved in tumors of different histogenesis: a
selective advantage for cancer cells? _Cancer Res_ 1998; 58: 815–822. CAS PubMed Google Scholar * Roy J, Vantol B, Cowley EA, Blay J, Linsdell P . Pharmacological separation of hEAG and
hERG K+ channel function in the human mammary carcinoma cell line MCF-7. _Oncol Rep_ 2008; 19: 1511–1516. CAS PubMed Google Scholar * Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B _et
al_. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. _Cancer Res_ 2002; 62: 4843–4848. CAS PubMed Google Scholar * Afrasiabi E, Hietamaki M, Viitanen T, Sukumaran
P, Bergelin N, Tornquist K . Expression and significance of HERG (KCNH2) potassium channels in the regulation of MDA-MB-435S melanoma cell proliferation and migration. _Cell Signal_ 2010;
22: 57–64. Article CAS PubMed Google Scholar * Crociani O, Guasti L, Balzi M, Becchetti A, Wanke E, Olivotto M _et al_. Cell cycle-dependent expression of HERG1 and HERG1B isoforms in
tumor cells. _J Biol Chem_ 2003; 278: 2947–2955. Article CAS PubMed Google Scholar * Zhao J, Wei XL, Jia YS, Zheng JQ . Silencing of herg gene by shRNA inhibits SH-SY5Y cell growth _in
vitro_ and _in vivo_. _Eur J Pharmacol_ 2008; 579: 50–57. Article CAS PubMed Google Scholar * Cherubini A, Hofmann G, Pillozzi S, Guasti L, Crociani O, Cilia E _et al_. Human
ether-a-go-go-related gene 1 channels are physically linked to beta1 integrins and modulate adhesion-dependent signaling. _Mol Biol Cell_ 2005; 16: 2972–2983. Article CAS PubMed PubMed
Central Google Scholar * D’Amico M, Biagiotti T, Fontana L, Restano-Cassulini R, Lasagna N, Arcangeli A _et al_. HERG current sustains a cardiac-type action potential in neuroblastoma S
cells. _Biochem Biophys Res Commun_ 2003; 302: 101–108. Article PubMed Google Scholar * Meves H . Slowing of ERG current deactivation in NG108-15 cells by the histidine-specific reagent
diethylpyrocarbonate. _Neuropharmacology_ 2001; 41: 220–228. Article CAS PubMed Google Scholar * Pancrazio JJ, Ma W, Grant GM, Shaffer KM, Kao WY, Liu QY _et al_. A role for inwardly
rectifying K+ channels in differentiation of NG108-15 neuroblastoma x glioma cells. _J Neurobiol_ 1999; 38: 466–474. Article CAS PubMed Google Scholar * Hofmann G, Bernabei PA, Crociani
O, Cherubini A, Guasti L, Pillozzi S _et al_. HERG K+ channels activation during beta(1) integrin-mediated adhesion to fibronectin induces an up-regulation of alpha(v)beta(3) integrin in the
preosteoclastic leukemia cell line FLG 29.1. _J Biol Chem_ 2001; 276: 4923–4931. Article CAS PubMed Google Scholar * Agarwal JR, Griesinger F, Stuhmer W, Pardo LA . The potassium
channel Ether a go-go is a novel prognostic factor with functional relevance in acute myeloid leukemia. _Mol Cancer_ 2010; 9: 18. Article PubMed PubMed Central Google Scholar * Rosati B,
Marchetti P, Crociani O, Lecchi M, Lupi R, Arcangeli A _et al_. Glucose- and arginine-induced insulin secretion by human pancreatic beta-cells: the role of HERG K(+) channels in firing and
release. _FASEB J_ 2000; 14: 2601–2610. Article CAS PubMed Google Scholar * Pond AL, Scheve BK, Benedict AT, Petrecca K, Wagoner RD, Shrier A _et al_. Expression of distinct ERG proteins
in rat, mouse, and human heart. Relation to functional I(Kr) channels. _J Biol Chem_ 2000; 275: 5997–6006. Article CAS PubMed Google Scholar * Bauer CK, Wulfsen I, Schäfer R, Glassmeier
G, Wimmers S, Flitsch J _et al_. HERG K(+) currents in human prolactin-secreting adenoma cells. _Pflugers Arch_ 2003; 445: 589–600. Article CAS PubMed Google Scholar * Staudacher I,
Wang L, Wan X, Obers S, Wenzel W, Tristram F _et al_. hERG K+ channel-associated cardiac effects of the antidepressant drug desipramine. _Naunyn Schmiedebergs Arch Pharmacol_ 2011; 383:
119–139. Article CAS PubMed Google Scholar * Crociani O, Cherubini A, Piccini E, Polvani S, Costa L, Fontana L _et al_. Erg gene(s) expression during development of the nervous and
muscular system of quail embryos. _Mech Dev_ 2000; 95: 239–243. Article CAS PubMed Google Scholar * Binggeli R, Weinstein RC . Membrane potentials and sodium channels: hypotheses for
growth regulation and cancer formation based on changes in sodium channels and gap junctions. _J Theor Biol_ 1986; 123: 377–401. Article CAS PubMed Google Scholar * Ishihara K, Hiraoka M
. Gating mechanism of the cloned inward rectifier potassium channel from mouse heart. _J Membr Biol_ 1994; 142: 55–64. Article CAS PubMed Google Scholar * Wonderlin WF, Strobl JS .
Potassium channels, proliferation and G1 progression. _J Membr Biol_ 1996; 154: 91–107. Article CAS PubMed Google Scholar * Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy
KW _et al_. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. _Cell_ 1999; 97: 175–187. Article CAS PubMed Google Scholar * Roepke TK, Purtell K,
King EC, La Perle KM, Lerner DJ, Abbott GW . Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. _PLoS One_ 2010; 5: e11451. Article PubMed PubMed Central
Google Scholar * Jones EMC, Roti Roti EC, Wang J, Delfosse SA, Robertson GA . Cardiac IKr channels minimally comprise hERG 1a and 1b subunits. _J Biol Chem_ 2004; 279: 44690–44694. Article
CAS PubMed Google Scholar * Li H, Liu L, Guo T, Zhang J, Li X, Du W _et al_. Expression and functional role of HERG1, K+ channels in leukemic cells and leukemic stem cells. _J Huazhong
Univ Sci Technolog Med Sci_ 2007; 27: 257–260. Article CAS PubMed Google Scholar * Fernando MA, Heaney AP . Alpha1-adrenergic receptor antagonists: novel therapy for pituitary adenomas.
_Mol Endocrinol_ 2005; 19: 3085–3096. Article CAS PubMed Google Scholar * Borowiec AS, Hague F, Harir N, Guenin S, Guerineau F, Gouilleux F _et al_. IGF-1 activates hEAG K(+) channels
through an Akt-dependent signaling pathway in breast cancer cells: role in cell proliferation. _J Cell Physiol_ 2007; 212: 690–701. Article CAS PubMed Google Scholar * Benning CM,
Kyprianou N . Quinazoline-derived alpha1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an alpha1-adrenoceptor-independent action. _Cancer Res_ 2002; 62: 597–602. CAS
PubMed Google Scholar * Thomas D, Bloehs R, Koschny R, Ficker E, Sykora J, Kiehn J _et al_. Doxazosin induces apoptosis of cells expressing hERG K+ channels. _Eur J Pharmacol_ 2008; 579:
98–103. Article CAS PubMed Google Scholar * Davey M . Mechanism of alpha blockade for blood pressure control. _Am J Cardiol_ 1987; 59: 18G–28G. Article CAS PubMed Google Scholar *
The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the
antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). _JAMA_ 2000; 283: 1967–1975. Article Google Scholar * Kang PM, Izumo S . Apoptosis and heart failure:
a critical review of the literature. _Circ Res_ 2000; 86: 1107–1113. Article CAS PubMed Google Scholar * Kyprianou N, Vaughan TB, Michel MC . Apoptosis induction by doxazosin and other
quinazoline alpha1-adrenoceptor antagonists: a new mechanism for cancer treatment? _Naunyn Schmiedebergs Arch Pharmacol_ 2009; 380: 473–477. Article CAS PubMed PubMed Central Google
Scholar * Partin JV, Anglin IE, Kyprianou N . Quinazoline-based alpha 1-adrenoceptor antagonists induce prostate cancer cell apoptosis via TGF-beta signalling and I kappa B alpha induction.
_Br J Cancer_ 2003; 88: 1615–1621. Article CAS PubMed PubMed Central Google Scholar * Eiras S, Fernandez P, Pineiro R, Iglesias MJ, Gonzalez-Juanatey JR, Lago F . Doxazosin induces
activation of GADD153 and cleavage of focal adhesion kinase in cardiomyocytes en route to apoptosis. _Cardiovasc Res_ 2006; 71: 118–128. Article CAS PubMed Google Scholar * Oyadomari S,
Mori M . Roles of CHOP/GADD153 in endoplasmic reticulum stress. _Cell Death Differ_ 2004; 11: 381–389. Article CAS PubMed Google Scholar * Kornberg LJ . Focal adhesion kinase and its
potential involvement in tumor invasion and metastasis. _Head Neck_ 1998; 20: 745–752. Article CAS PubMed Google Scholar * Walden PD, Globina Y, Nieder A . Induction of anoikis by
doxazosin in prostate cancer cells is associated with activation of caspase-3 and a reduction of focal adhesion kinase. _Urol Res_ 2004; 32: 261–265. Article CAS PubMed Google Scholar *
Zhao H, Lai F, Nonn L, Brooks JD, Peehl DM . Molecular targets of doxazosin in human prostatic stromal cells. _Prostate_ 2005; 62: 400–410. Article CAS PubMed Google Scholar * Pillozzi
S, Brizzi MF, Bernabei PA, Bartolozzi B, Caporale R, Basile V _et al_. VEGFR-1 (FLT-1), beta1 integrin, and hERG K+ channel for a macromolecular signaling complex in acute myeloid leukemia:
role in cell migration and clinical outcome. _Blood_ 2007; 110: 1238–1250. Article CAS PubMed Google Scholar * Shao XD, Wu KC, Hao ZM, Hong L, Zhang J, Fan DM . The potent inhibitory
effects of cisapride, a specific blocker for human ether-a-go-go-related gene (HERG) channel, on gastric cancer cells. _Cancer Biol Ther_ 2005; 4: 295–301. Article CAS PubMed Google
Scholar * Thomas D, Wendt-Nordahl G, Röckl K, Ficker E, Brown AM, Kiehn J . High-affinity blockade of HERG human cardiac potassium channels by the novel antiarrhythmic drug BRL-32872. _J
Pharmacol Exp Ther_ 2001; 297: 753–761. CAS PubMed Google Scholar * Thomas D, Gut B, Wendt-Nordahl G, Kiehn J . The antidepressant drug fluoxetine is an inhibitor of human
ether-a-go-go-related gene (HERG) potassium channels. _J Pharmacol Exp Ther_ 2002; 300: 543–548. Article CAS PubMed Google Scholar * Zhang S, Zhou Z, Gong Q, Makielski JC, January CT .
Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. _Circ Res_ 1999; 84: 989–998. Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS This study was supported in part by research grants from the ADUMED foundation (to DT), the German Heart Foundation/German Foundation of Heart Research (to DT), and the
Max-Planck-Society (TANDEM project to PAS). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Cardiology, Medical University Hospital, Heidelberg, Germany J Jehle, P A Schweizer, H
A Katus & D Thomas Authors * J Jehle View author publications You can also search for this author inPubMed Google Scholar * P A Schweizer View author publications You can also search
for this author inPubMed Google Scholar * H A Katus View author publications You can also search for this author inPubMed Google Scholar * D Thomas View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to D Thomas. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest.
ADDITIONAL INFORMATION Edited by V De Laurenzi RIGHTS AND PERMISSIONS This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To
view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jehle, J., Schweizer, P., Katus, H. _et
al._ Novel roles for hERG K+ channels in cell proliferation and apoptosis. _Cell Death Dis_ 2, e193 (2011). https://doi.org/10.1038/cddis.2011.77 Download citation * Received: 20 May 2011 *
Revised: 14 July 2011 * Accepted: 18 July 2011 * Published: 18 August 2011 * Issue Date: August 2011 * DOI: https://doi.org/10.1038/cddis.2011.77 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * hERG * K+ channel * cell proliferation * apoptosis * anticancer therapy