Functional and biochemical characterization of the baculovirus caspase inhibitor mavip35
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Many viruses express proteins which prevent the host cell death that their infection would otherwise provoke. Some insect viruses suppress host apoptosis through the expression of
caspase inhibitors belonging to the P35 superfamily. Although a number of P35 relatives have been identified, _Autographa californica_ (Ac) P35 and _Spodoptera littoralis_ (Spli) P49 have
been the most extensively characterized. AcP35 was found to inhibit caspases via a suicide substrate mechanism: the caspase cleaves AcP35 within its ‘reactive site loop’ then becomes
trapped, irreversibly bound to the cleaved inhibitor. The _Maruca vitrata_ multiple nucleopolyhedrovirus encodes a P35 family member (MaviP35) that exhibits 81% identity to AcP35. We found
that this relative shared with AcP35 the ability to inhibit mammalian and insect cell death. Caspase-mediated cleavage within the MaviP35 reactive site loop occurred at a sequence distinct
from that in AcP35, and the inhibitory profiles of the two P35 relatives differed. MaviP35 potently inhibited human caspases 2 and 3, DCP-1, DRICE and CED-3 _in vitro_, but (in contrast to
AcP35) only weakly suppressed the proteolytic activity of the initiator human caspases 8, 9 and 10. Although MaviP35 inhibited the AcP35-resistant caspase DRONC in yeast, and was sensitive
to cleavage by DRONC _in vitro_, MaviP35 failed to inhibit the proteolytic activity of bacterially produced DRONC _in vitro_. SIMILAR CONTENT BEING VIEWED BY OTHERS STRUCTURAL BASIS FOR THE
NON-SELF RNA-ACTIVATED PROTEASE ACTIVITY OF THE TYPE III-E CRISPR NUCLEASE-PROTEASE CRASPASE Article Open access 07 December 2022 DUAL ROLES AND EVOLUTIONARY IMPLICATIONS OF P26/POXIN IN
ANTAGONIZING INTRACELLULAR CGAS-STING AND EXTRACELLULAR MELANIZATION IMMUNITY Article Open access 14 November 2022 DIRECT CLEAVAGE OF CASPASE-8 BY HERPES SIMPLEX VIRUS 1 TEGUMENT PROTEIN
US11 Article Open access 19 July 2022 MAIN Cell death is essential for normal animal development and to destroy pre-cancerous and auto-immune cells, but it has been postulated that apoptosis
originally evolved to defend primitive multicellular organisms against intracellular pathogens such as viruses.1 Over evolutionary time, an ‘arms race’ has developed between viruses and
their hosts. Cellular machineries detect infection and activate self-destruction pathways, limiting the ability of the virus to replicate and spread to other cells. Viruses, in turn, have
evolved ways to suppress their hosts’ apoptotic machineries during the early phase of infection. Targeting caspases is one approach adopted by viruses to block their host cells’ suicidal
reaction to infection.2 The P35 family is a group of caspase inhibitors encoded by viruses that infect insects. Almost all of the viruses that possess P35 relatives are baculoviruses:3 the
sole exception known to date is the _Amsacta moorei_ entomopoxvirus.4 No cellular P35 homologs have been described as yet, although as baculoviruses usually derive their genes from their
hosts,5 it seems likely that P35 genes did evolve from a cellular ancestor. The best-studied P35 family member is AcP35, encoded by the baculovirus _Autographa californica_ multi
nucleopolyhedrovirus (AcMNPV).6 It inhibits caspases via a substrate trap mechanism.7, 8, 9 The caspase cleaves AcP35 within the reactive site loop. This cleavage provokes a conformational
change within the inhibitor, targeting its amino terminus to the caspase's active site, preventing hydrolysis of a thioester adduct between the inhibitor and the protease, and thus
locking the caspase in an inactive, P35-bound form.7 Of the many mammalian, insect and nematode caspases tested, very few were found to be insensitive to AcP35. The _Drosophila_ initiator
caspase DRONC was shown to be resistant to inhibition by AcP35.10, 11 Processing of downstream _Spodoptera_ caspases proceeded in the presence of AcP35,12 implying that a _Spodoptera_ DRONC
ortholog (denoted ‘Sf-caspase-X’) is also resistant to AcP35 inhibition. AcP35 could inhibit the enzymatic activity of recombinant caspase 9 (DRONC's mammalian counterpart), however
extremely high concentrations of AcP35 were required to prevent apoptosome-activated caspase 9 from cleaving its physiological substrate, caspase 3.13 This suggests that AcP35 cannot
efficiently interfere with the function of naturally activated caspase 9. _Bombyx mori_ nucleopolyhedrovirus (BmNPV) encodes a protein (BmP35), which shares 91% of its amino-acid sequence
with AcP35. BmP35 displayed only weak anti-apoptotic activity14 and, unlike AcP35, BmP35 was dispensable for normal viral propagation.15, 16 Extracts from mammalian cells expressing BmP35
were less potent than lysates from AcP35-expressing cells at inhibiting recombinant caspase 3, although lower BmP35 expression levels may have contributed to this difference.13 No
quantitative data have been published regarding the caspase inhibitory potency or specificity of BmP35, and no other close relatives of AcP35 have been functionally or biochemically
investigated to date. Some baculoviruses encode distant relatives of AcP35, which constitute the P49 subfamily. _Spodoptera littoralis_ (Spli) NPV-P49 is the best-studied member of this
subfamily. Like AcP35, SpliP49 is a broad-spectrum caspase inhibitor that could suppress insect17, 18, 19, 20 and mammalian21 cell death. Unlike AcP35, SpliP49 could inhibit DRONC-mediated
yeast lethality,21 but it was incapable of preventing DRICE processing in _Drosophila_ cells.19 SpliP49 could, however, prevent processing of executioner _Spodoptera_ caspases,18, 20
implying that it can inhibit the proposed Sf-caspase-X. AcP35 contains the cleavage sequence DQMD’G within its reactive site loop, but SpliP49 instead possesses the sequence TVTD’G at this
position. This sequence is required for SpliP49 to inhibit the distal insect caspase Sf-caspase-X, but its insertion into the AcP35 reactive site loop failed to confer this capability,20
indicating that other regions of the SpliP49 protein, not shared by AcP35, are critical for its ability to inhibit insect initiator caspases. The caspase inhibitor AMVP33 from _Amsacta
moorei_ entomopoxvirus is the least homologous member of the P35 superfamily, exhibiting only 25% amino acid identity to AcP35.4 The baculovirus _Maruca vitrata_ (_Mavi_) MNPV infects the
legume pod borer _Maruca vitrata_, and may offer a biological means of controlling this important pest of legume crops.22 The recent sequencing of the _Mavi_MNPV genome23 revealed the
presence of a P35 ortholog (MaviP35). The predicted MaviP35 protein is highly homologous to AcP35, but its predicted reactive site loop possesses a distinct caspase cleavage sequence. Here,
we report our characterization of the apoptosis and caspase inhibitory properties of MaviP35. RESULTS Sequencing of the _Mavi_MNPV genome23 revealed that this virus encoded a P35 ortholog
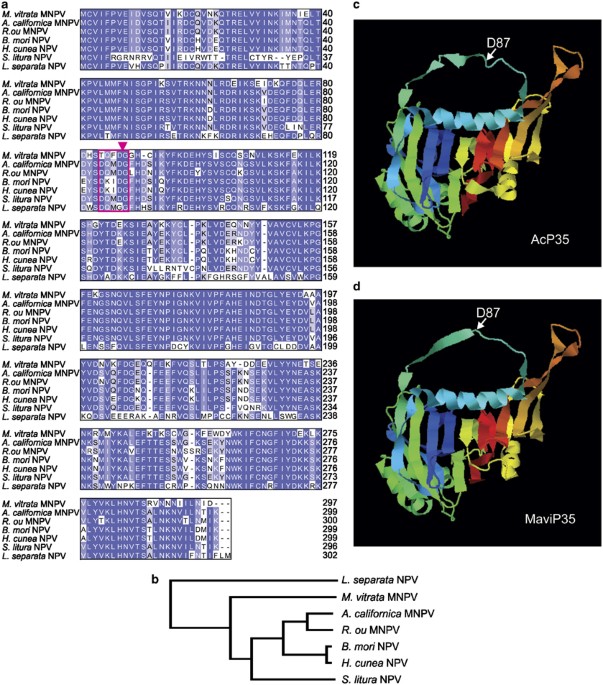
that was 81% identical to the founding member of this family, AcP35 (Figures 1a and b), and modeling suggested the two relatives may adopt similar structures (Figures 1c and d). Residues
determined to be essential for the caspase inhibitory activities of AcP35, including C28 and D87,24, 25 were conserved in MaviP35, suggesting it too may function as a caspase inhibitor that
could prevent apoptosis. We tested this hypothesis by overexpressing MaviP35 in mammalian and insect cells, and monitoring the transfectants’ sensitivity to apoptosis. MaviP35 inhibited
insect cell death and caspase activity triggered by infection with an AcP35-deficient baculovirus, although less efficiently than AcP35 (Figures 2a and b). MaviP35 and AcP35 inhibited insect
cell death induced by actinomycin D to a similar extent (Figure 2c), but MaviP35 protected a larger proportion of insect cells than AcP35 against apoptosis induced by UV irradiation (Figure
2d). MaviP35 and AcP35 afforded similar levels of protection to mammalian cells against cisplatin-induced apoptosis (Figure 2e), but MaviP35 was less protective than AcP35 against death
induced by TNF-related apoptosis-inducing ligand (TRAIL; Figure 2f). We have previously exploited yeast-based assays to visualize caspase activity and inhibition, and these were used to
provide an indication of the specificity of MaviP35 for various caspases. MaviP35 protected yeast from death induced by mammalian caspases 1, 2, 3, 5, 7 and 8, the _Drosophila_ caspases
DCP-1 and DRICE, and CED-3 from _Caenorhabditis elegans_ (Figure 3). In this system, MaviP35 appeared to exhibit similar activity to AcP35, and protected yeast from death induced by caspases
5, 8 and CED-3 better than SpliP49 (Figure 3). AcP35 has been shown to inhibit caspases via a pseudosubstrate mechanism, and mutation of the caspase cleavage site abolishes caspase and
apoptosis inhibitory activity.7, 8 Comparison of the MaviP35 and AcP35 sequences predicted that caspases may cleave MaviP35 after residue D87 within the site TQFD87′G (Figure 1a). Consistent
with this residue being critical for caspase inhibition, a putative P1 mutant failed to prevent DRICE induced yeast death (Figure 4). Lysates from yeast expressing active DRICE exhibited
considerable DEVDase activity. Co-expression of untagged or carboxyl terminally FLAG-tagged wild-type MaviP35 or AcP35 abolished this activity, but their cleavage site mutants had negligible
impact (Figure 4). Immunoblotting confirmed that the tagged mutants were expressed at least as abundantly as their wild-type counterparts. Interestingly, cleavage products could not be
detected in lysates from yeast co-expressing DRICE with either MaviP35-FLAG or AcP35-FLAG. Because DRICE activity in this system is generated through autoactivation, we suspect that only
relatively few P35 molecules would be required to block this feedback loop, and immunoblotting may not be sufficiently sensitive to detect this small number of cleaved proteins. The yeast
system is a sensitive tool for observing caspase inhibition within a naive eukaryotic environment, but only provides limited insight into the strength of inhibition. To gain a quantitative
understanding of MaviP35's caspase inhibitory activity, we purified FLAG-tagged MaviP35 and AcP35 and examined their ability to prevent recombinant caspases from cleaving fluorogenic
substrates _in vitro_. When present at 100–1000-fold excess, MaviP35 diminished by at least 90% the activity of caspases 2, 3, DRICE, DCP-1 and CED-3. Weaker inhibition was seen for caspases
1 and 7. MaviP35 only partially reduced the activity of caspases 6, 8 and 9, and negligible inhibition of caspase 10 was observed (Figure 5). A particularly striking difference between
AcP35 and MaviP35 related to inhibition of caspase 8. As observed previously,26 AcP35 potently suppressed the proteolytic activity of this enzyme, yet a 100-fold excess of MaviP35 only
decreased its activity by about half (Figure 5). The alignment of P35 subfamily members revealed that the P4 and P2 residues differed between AcP35 and MaviP35 (P4-DQMD-P1 _versus_
P4-TQFD-P1, respectively). Mutagenesis studies of AcP35 had previously demonstrated that changing its P4 aspartate residue to either alanine or asparagine markedly impaired its ability to
inhibit caspases 3 and 8,7 highlighting the importance of the P4 amino acid for caspase inhibition. The cleavage site of MaviP35, containing a P4 threonine residue, was reminiscent of the
site at which DRONC auto-processes between its large and small subunit (TQTE)11 and, to a lesser extent, the caspase cleavage site within the SpliP49 reactive site loop (TVTD).17 This
prompted us to wonder whether MaviP35 may be the first example of a P35 subfamily member that can inhibit DRONC. Consistent with this notion, expression of MaviP35 completely abolished
DRONC-mediated yeast death (Figure 6a) and recombinant DRONC could cleave purified MaviP35, although not as efficiently as DRICE (Figures 6b and c). We therefore sought to determine whether
MaviP35 could suppress DRONC activity in insect cells. DRONC can cleave DRICE between its large and small subunits10, 11 and cleavage of DRICE in _Drosophila_ cells has previously been used
as a readout of DRONC activity.19, 27 A GFP-tagged active site mutant of DRICE expressed in _Drosophila_ Kc167 cells was completely processed to yield a 39-kDa product following actinomycin
D treatment (Figure 6d), as expected from DRONC cleavage between the large and small subunits of DRICE (Figure 6e). Enforced expression of DIAP1 completely inhibited cleavage of
DRICEC211A-eGFP in actinomycin D-treated _Drosophila_ cells (Figure 6). Curiously, AcP35 partially inhibited DRICEC211A-eGFP cleavage, with more than half of the protein remaining intact in
cells co-expressing AcP35. MaviP35 also partially inhibited this cleavage event, although less potently than AcP35. Purified MaviP35 did not impair the ability of recombinant DRONC to cleave
a peptide substrate (Figure 6f). Using a range of substrate and inhibitor concentrations, inhibition by bacterially produced DRONC of MaviP35 was extremely weak (Figure 7). Quantitation of
MaviP35's inhibition of other caspases confirmed the data shown in Figure 5: strong inhibition of executioner caspases, but weak to negligible inhibition of initiator caspases (Figure
7c). DISCUSSION This study describes the apoptosis and caspase inhibitory properties of a new P35 subfamily member: MaviP35. Like AcP35, MaviP35 could inhibit insect and mammalian cell
death, was susceptible to caspase cleavage, and could inhibit the proteolytic activity of caspases. Nevertheless, AcP35 and MaviP35 differed in their specificity profile. MaviP35 inhibited
executioner apoptotic caspases with similar potency to AcP35. However, MaviP35 was substantially less potent than AcP35 at inhibiting mammalian caspases 8 and 10. This may explain the weaker
protection afforded by MaviP35 relative to AcP35 against TRAIL-induced cell death. MaviP35 was also a weaker inhibitor of recombinant caspase 9 than AcP35. However, it is important to note
that published data suggest that the susceptibility of recombinant caspase 9 to AcP35 _in vitro_ is not mirrored by apoptosome-activated caspase 9 within cell lysates,13 so it is possible
that AcP35 and MaviP35 are both incapable of interfering with the activity of naturally activated caspase 9 _in vivo_. The P4 residue of MaviP35 (threonine) differs from that of AcP35
(aspartate). Mutation of P4 in AcP35 to asparagine reduced its ability to inhibit caspase 3 by 47-fold,7 yet MaviP35 – which contains the slightly larger polar uncharged residue threonine at
this position – inhibited caspase 3 with similar efficiency to wild-type AcP35. Presumably differences in other regions of the protein compensate, allowing MaviP35 to efficiently suppress
caspase 3 activity. The MaviP35 cleavage site resembles the auto-processing site between the large and small subunits of DRONC, and MaviP35 was sensitive to DRONC proteolysis _in vitro_.
MaviP35 inhibited yeast death triggered by high-level expression of DRONC, suggesting that it could function as a pseudo-substrate inhibitor of DRONC. However, two pieces of evidence argue
against this possibility. First, MaviP35 was an extremely weak inhibitor of recombinant DRONC activity _in vitro_. Its _K_i was indistinguishable from that of AcP35, which was incapable of
inhibiting DRONC in yeast and _in vivo_10, 11 and was resistant to DRONC cleavage _in vitro_. Second, MaviP35 only partially inhibited actinomycin D-induced cleavage of the DRICEC211A–eGFP
fusion protein, impeding processing to a lesser extent than AcP35. This result implies that actinomycin D treatment provoked DRONC-mediated (AcP35/MaviP35 resistant) processing of the
DRICE-eGFP substrate in Kc167 cells, but also suggested that other proteases, sensitive to AcP35/MaviP35 inhibition, also contributed to this proteolysis. Taken together, these data lead us
to postulate that MaviP35 functions as a classical substrate for DRONC, rather than as a suicide substrate inhibitor. It is possible that the ability of MaviP35 to suppress DRONC-mediated
yeast lethality reflects substrate competition: DRONC's proteolytic attention may be diverted to MaviP35 processing rather than cleavage of essential yeast proteins. Despite the high
homology between MaviP35 and AcP35, this study has revealed an important difference in their caspase specificity. Both inhibitors could suppress downstream caspases from insects and mammals
(and CED-3), but only AcP35 could efficiently block the activities of mammalian caspases-8 and -10. Neither AcP35 nor MaviP35 could significantly inhibit the _Drosophila_ initiator caspase
DRONC. MATERIALS AND METHODS SEQUENCE COMPARISONS AND STRUCTURAL MODELING Sequences of the P35 subfamily members were aligned using the ‘Multialign’ program28 and formatted using Jalview
2.0.29 Genbank accession numbers for the P35 sequences used were: _Mavi_MNPV: YP_950833, _Ac_MNPV: NP_054165.1, _Ro_MNPV: NP_703122, _Bm_NPV: AAO12972, _Hycu_NPV: AAO17287, S_plt_NPV:
CAA71304 and _Lese_NPV: AAF78504. Protein Homology/analogY Recognition Engine (Phyre) was used to predict the structure of MaviP35.30 AcP35 and predicted MaviP35 structures were depicted
using Jmol: an open-source Java viewer for chemical structures in 3D (http://www.jmol.org/). PLASMID CONSTRUCTION For yeast experiments, coding DNA sequences were expressed from inducible
Gal1/10 promoters.31 Plasmids-expressing caspase 2, caspase 3-lacZ, caspase 5, caspase 753, caspase 8, CED-3, reverse DRICE, DCP-1, DRONC, AcP35, AcP35-F, AcP35D87A-F and SpliP49-F have been
described previously.11, 21, 32, 33, 34 Other plasmids were generated as follows: the caspase 1 coding region was amplified using oligonucleotides 1 and 2 from pET21b-Casp-1-His (purchased
from Addgene Cambridge, MA, USA). The product was cut with _Bam_HI/_Xba_I and ligated into pGALL-(_LEU2_)32 cut with _Bam_HI/_Xba_I. The _MaviP35_ coding region was amplified with
oligonucleotides 3 and 4 from a plasmid kindly donated by Prof. Chung-Hsiung Wang, cut with _Bam_HI/_Xho_I and ligated into pGALL-(_HIS3_)32 cut with _Bam_HI/_Xho_I. _MaviP35_ was also
amplified with oligonucleotides 3 and 5 and cloned the same way into pGALL-(_HIS3_), to incorporate a carboxyl terminal FLAG tag. Site-directed mutagenesis to generate _MaviP35__D87A_ was
performed using PCR. After excising _MaviP35_ from pGALL-(_HIS3_)-MaviP35 and inserting it into Bluescript II SK+ via _Bam_HI/_Xho_I, a PCR was conducted using _MaviP35-F_ as a template
(oligonucleotides 6 and 7) to amplify the 3′ portion of the gene incorporating a P1 mutation. This product was cut with _BclI_/_Xho_I and inserted into _BclI_/_Xho_I cut MaviP35-Bluescript
II SK+. The resulting P1-mutated _MaviP35-F_ gene was then excised with _Bam_HI/_Xho_I and ligated into pGALL-(_HIS3_). For mammalian and insect cell expression, _MaviP35-F_ was amplified
from pGALL-(_HIS3_)-MaviP35-F with oligonucleotides 8 and 7, then cut with _Bam_HI/_Xho_I, blunted and inserted into pHSP70PLVI+CAT (chloramphenicol acetyl transferase)35 cut with
_BglII_/_EcoR_I blunted or pEF36 cut with _Bam_HI/_Xba_I blunted. _Caspase 3_ was amplified using primers 9 and 10, then digested with _Nde_I/_Xho_I and ligated into _NdeI_/_Xho_I cut
pET23a. All amplified fragments were sequenced to verify the absence of any unintentional mutations. The pAct5eGFP vector was kindly provided by Samuel Le Fort and David Vaux. The coding
sequencing of _DRICE__C211A_ was amplified from pGMR-DRICEC211A (described below) using primers 11 and 12, cut with _BglII_ and cloned into pACT5eGFP cut with _Bam_HI to generate
pACT5c-DRICEC211A-eGFP. Restriction analyses and sequencing were performed to determine the orientation of the insert and to check the sequence was correct. pGMR-DRICE was made by amplifying
_DRICE_ with primers 13 and 14, then cutting the product with _Bgl_II and _Xba_I and cloning into pGMR.37 pGMR-DRICEC211A was constructed by amplifying the 3′ portion of _DRICE_ encoding
the active site with a mutagenic forward primer (15) and wild-type reverse primer (16), digesting the product with _Nhe_I and _Not_I and ligating into pGMR-DRICE cut with _Nhe_I and _Not_I.
The DNA encoding eGFP was removed from pAct5eGFP by cutting with _Bam_HI and _Xba_I, blunting using Klenow polymerase and religating to yield pAct5c. _Bam_HI/_Xba_I fragments encoding either
DIAP1 (obtained by digesting pGALL-(_HIS3_)-DIAP1) or MaviP35-FLAG (excised from pGALL-(_HIS3_)-MaviP35-F) were ligated into _Bam_HI/_Xba_I cut pAct5c-eGFP, replacing the _eGFP_ gene. The
sequences of the nucleotides referred to above were as follows: * 1:: 5′-CGGGATCCATGGCCGACAAGGTCCTGAAGGAG-3′ * 2:: 5′-GCTCTAGATTAATGTCCTGGGAAGAGGTAGAAACATC-3′ * 3::
5′-GGGATCCCATATGTGTGTAATTTTTCCAGTAG-3′ * 4:: 5′-GCCTCGAGTTAATCAATGTTTAATATTATATTG-3′ * 5:: 5′-GCCTCGAGTTACTTGTCATCGTCGTCCTTGTAGTCCATATCAATGTTTAATATTA TATTGTTG-3′ * 6::
5′-CAATTTGATCAACTAGAACGCGACCACAGCACTCAATTCGCT GGAGGCC-3′ * 7:: 5′-CTTTATTATTTTTATTTTATTGAGAGGGTGG-3′ * 8:: 5′-GCGGATCCGCCATGTGTGTAATTTTTCCAGTAG-3′ * 9::
5′-GGAATTCCATATGGAGAACACTGAAAACTCAGTGG-3′ * 10:: 5′-CCCTCGAGGTGATAAAAATAGAGTTCTTTTGTGAGC-3′ * 11:: 5′-GTCAGATCTCAAAATGGACGCCACTAACAATGGAG-3′ * 12:: 5′-GTCAGATCTACCCGTCCGGCTGGAGCCAAC-3′ *
13:: 5′-CGAGATCTCCGCCATGGACGCCACTAACAATGGAGAATCC-3′ * 14:: 5′-CGTCTAGACTAAACCCGTCCGGCTGGAGCCAACTGC-3′ * 15:: 5′-CCTCGCTAGCCGGCAAACCCAAGTTGTTCTTCATACAGGCCGCCCAGGGC-3′ * 16::
5′-GCACTAGTGCGGCCGCCTAAACCCGTCCGGCTGGAGCCAACTGC-3′ APOPTOSIS ASSAYS FROM INSECT CELLS Sf21 cells were plated at 8 × 105 cells per well in six-well plates in TC-100 insect medium (Invitrogen,
Carlsbad, CA, USA) plus 10% fetal bovine serum (FBS; Atlanta Biologicals, Atlanta, GA, USA), and allowed to attach overnight at 27°C. Transfections were performed using lipofectin, which
was prepared as a 1.5 : 1 mixture of DOTAP ((_N_-(1-(2,3-Dioleoyloxy)propyl)-_N_,_N_,_N_,-trimethylammonium chloride salt; Avanti Polar Lipids, Alabaster, AL, USA) and DOPE (L-α
Phosphatidylethanolamine, dioleoyl; Sigma-Aldrich, St. Louis, MO, USA). For each well, 2.5 _μ_g of the eGFP expression plasmid pHSP70GFPBsu36I38 was mixed with 2.5 _μ_g of either
pHSP70PLVI+CAT, pHSP70PLVI+AcP35 or pHSP70PLVI+MaviP35F. This DNA was diluted to 100 _μ_l with TC-100 lacking FBS and incubated for 5 min at room temperature (RT). In a separate tube, 6 _μ_l
lipofectin was diluted to 100 _μ_l with TC-100 lacking FBS and incubated for 5 min at RT. The mixtures of DNA and lipofectin were then combined and allowed to incubate for 15 min at RT.
During this incubation the Sf21 cells were washed twice with 1 ml of TC-100 lacking FBS. After the last wash, 800 _μ_l of TC-100 lacking FBS was left in each well and the DNA/lipofectin
mixture (200 _μ_l) was added to each well and allowed to incubate at 27°C for 4 h. The mixture plus the media were removed and 2 ml of TC-100 plus 10% FBS was added to each well. The cells
were heat shocked 24 h post transfection at 42°C for 30 min, to drive expression from the hsp70 promoter. The cells were then induced to undergo apoptosis 4 h post heat shock, by treatment
with either UV (by placing the plates on a transilluminator for 10 min) or actinomycin D (Invitrogen; 250 ng/ml). To determine cell viability, the number of GFP-expressing cells was counted
in each well both immediately before and 17 h after UV or actinomycin D treatment. Three random fields of view per sample were counted per well, and three separate wells were assayed per
treatment. To assay sensitivity to infection-mediated apoptosis, Sf9 cells (106) were plated in six-well culture dishes for 2 h in the TC-100 medium with 10% FBS. After 2 h, the medium was
replaced with Grace's insect unsupplemented medium (Invitrogen). Cells were transiently transfected with pHSP70PLVI+CAT, pHSP70PLVI+AcP35 or pHSP70PLVI+MaviP35F using 3 _μ_g of each
plasmid and 6 _μ_l of lipofectin. Transfection mixtures were replaced with TC-100 plus 10% FBS after 5 h incubation with cells. Cells were infected at 24 h post transfection with
vAcP35KO-PG39 at a multiplicity of infection of 1 PFU/cell, and then harvested at 48 h post infection for caspase and viability assays. Caspase assays were performed using the substrate
Ac-DEVD-AFC (MP Biomedicals, Solon, OH, USA) as described previously.40 To assess viability, three random fields of view were photographed ( × 200 magnification), and viable cells were
counted for each well. Cell viability was determined by counting the non-apoptotic cells and comparing to the number of viable cells in a mock-infected control at 0 h post infection, which
was set at 100%. DRICE CLEAVAGE ASSAYS IN KC167 CELLS Two million Kc167 cells (kindly provided by Gary Hime) were transfected with 0.2 _μ_g of either pAct5c-eGFP or pAct5c-DRICE-eGFP plus
1.8 _μ_g of either pAct5c, pAct5c-MaviP35-F or pAct5c-DIAP1, using the Effectene transfection reagent (Qiagen, Doncaster, Victoria, Australia) according to the manufacturer's
instructions. After 24 h transfection, the cells were incubated in media containing 0 or 1 _μ_M of actinomycin D for 12 h. Cells were lysed in mammalian lysis buffer (50 mM Tris pH 7.5, 375
mM NaCl, 1 mM ethylenediamine tetra-acetic acid, 1% Triton X-100) containing protease inhibitors (protease inhibitor cocktail set 1; Calbiochem, Darmstadt, Germany) and subjected to 12%
SDS-PAGE and either Coomassie stained or immunoblotted using anti-GFP (Roche Applied Science no. 11814460001; Castle Hill, New South Wales, Australia) or anti-FLAGM2 (Sigma no. F3165) and
anti-mouse IgG-HRP (Sigma no. A9044). MAMMALIAN APOPTOSIS ASSAYS SV-40 transformed mouse embryonic fibroblasts (MEFs) were co-transfected with 1 _μ_g of CMV-lacZ and either 3 _μ_g of pEF,
AcP35-pEF or MaviP35-pEF using FuGENE HD transfection reagent (Roche; Basel, Switzerland). LN18 glioblastoma cells (ATCC; Manassas, VA, USA) were co-transfected with 1 _μ_g of CMV-lacZ and
either 2 _μ_g of pEF, AcP35-pEF or MaviP35-pEF using Lipofectamine (Invitrogen). Transfections were performed according to the manufacturers’ instructions. Twenty-four hours after
transfection, the medium was removed and the cells were incubated with fresh unsupplemented media or media containing cisplatin (Mayne Pharma, Mulgrave, Victoria, Australia) or Superkiller
(crosslinked) TRAIL (Alexis Biochemicals, Lausen, Switzerland). After 24 h, the cells were stained with 5-bromo-4-chloro-3-indolyl-_β_-D-galactopyranoside and the blue cells were scored for
viable _versus_ apoptotic morphology, as previously published.36 YEAST TRANSFORMATION AND DEATH ASSAYS _Saccharomyces cerevisiae_ yeast strain W303_α_ was transformed31 and analyzed in
survival assays21 as described previously.31 Caspase 1 was expressed using the pGALL-(LEU2)-Casp1 vector described above. PROTEIN ASSAYS FROM YEAST Yeast transformants were grown and
transgene expression induced as described previously.11 The lysates were subjected to SDS-PAGE and the gels were then stained with Coomassie brilliant blue (Sigma) to visualize protein
loading and immunoblotted as described previously.11 The membranes were probed with an antibody recognizing the FLAG tag (clone M2; Sigma) and anti-mouse-HRP (Sigma). To measure the caspase
activity via fluorescence analysis, the yeast were treated as follows: An overnight culture was pelleted, washed twice with 1 ml of TE (Tris HCl 10 mM pH 8, EDTA 1 mM) and induced for 6.5 h
in complete media containing 2% galactose. After pelleting the yeast culture, the yeast was weighed and glass beads were added. To lyse the cells, 5 ml of CelLyticY reagent (Sigma) with 10
mM DTT was added per 1 g of yeast cells. After gently shaking the cells for 30 min at RT, the debris was removed by centrifugation at 16 100 × _g_ for 10 min at 4°C. The protein
concentration of the supernatant was measured using the Bicinchoninic acid protein assay kit (Sigma). In fluorescence assays, lysate (0.5 mg/ml) was mixed with Ac-DEVD-AFC (100 _μ_M) in
DRICE activity buffer (50 mM HEPES pH7.5, 10% sucrose, 0.1% CHAPS, 5 mM DTT, 100 mM NaCl, 1 mM EDTA), and the fluorescence (excitation 410 nm, emission 500 nm) was measured every 30 s for 1
h. The slope of each curve was calculated using Graphpad Prism 5.0 (La Jolla, CA, USA). The slope of each curve determines the concentration of free AFC per minute, which provides a measure
of caspase activity. PROTEIN PURIFICATION FROM YEAST Transformants were grown in 5 ml glucose-containing selective medium to stationary phase, then expanded by addition of 195 ml
glucose-containing selective medium. After expanding the cells for 16–18 h, the pelleted yeast were washed once with 100 ml TE and resuspended in 1 l of galactose-containing complete media
for 6.5 h induction. Cells were harvested at 12 100 × _g_, 4°C for 15 min then glass beads were added after weighing the pellets. To lyse the cells CelLyticY reagent (Sigma) was used as
described above. The supernatant was incubated for 30 min at 4°C with 200 _μ_l Anti-FLAG M2 affinity gel (Sigma), which had been previously washed three times with 12 × resin volume washing
buffer (Tris HCl 50 mM pH 7.4, 150 mM NaCl). Incubated beads were pelleted 5 min at 3750 g, RT then washed with 32 resin volumes of washing buffer for 10 min at 4°C. Five elution fractions
were collected each using one resin volume of elution buffer (1 mM HEPES pH 7.0, 0.1% PEG, 0.001% CHAPS, 0.1 mM DTT, 200 ng/_μ_l FLAG peptide (Sigma)). For each elution step the beads were
incubated for 2 min at 4°C with agitation, and supernatant was collected after a pelleting for 1 min at 16 100 g, 4°C. After subsequent SDS-PAGE analysis and Coomassie brilliant blue (Sigma)
staining, the fractions containing pure FLAG-tagged proteins were pooled, and protein concentration was determined using the Bio-Rad Protein Assay (Bio-Rad, Gladesville, New South Wales,
Australia). RECOMBINANT CASPASES Caspases 1, 2, 6, 7, 8, 9 and 10 were purchased from Enzo Life Sciences (Farmingdale, NY, USA). BL21-(DE3)-pLysS (Merck, Darmstadt, Germany) bacteria were
transformed with caspase 3-pET23a (described above) or the following previously published plasmids: DCP-1-pET23a,32 DRICE-pET23a,32 CED-3-pET23a,34 DRONC-pET23a.11 Caspase 3, DCP-1, DRICE
and CED-3 were purified as described previously.34 DRONC purification was carried out as follows: A transformant colony was inoculated into 1.5 ml 2YT-amp/chlor (16 g/l tryptone, 10 g/l
yeast extract, 5 g/l NaCl, 100 _μ_g/ml ampicillin and 35 _μ_g/ml chloramphenicol) and grown overnight at 37°C. A volume of 1 ml of this culture was expanded into 50 ml of pre-warmed
2YT-amp/chlor and grown at 37°C for 2 h, 200 r.p.m. A total of 10 ml of this was mixed with 190 ml of pre-warmed 2YT amp/chlor in a 2-l baffled flask and shaken at 37°, 200 r.p.m. until
OD600 reached 0.6–0.8. IPTG was added to a final concentration of 1 mM and the culture was shaken at 20°C for 18 h, then pelleted for 10 min at 3000 g, 4°C and then frozen at −80°C. The
pellet was thawed then resuspended in a 10-ml Bug Buster Mastermix (Merck) by pipetting, then incubated for 20 min at RT. Insoluble cell debris was removed by centrifugation at 16 100 × _g_
for 20 min at 4°C. Half a milliliter of NiNTA resin (Qiagen) was washed twice in phosphate buffer (50 mM NaHPO4, 300 mM NaCl), then incubated with the induced bacterial lysate for 30 min at
4°C, gently mixing. The beads were washed twice with phosphate buffer containing 5 mM imidazole, then the caspase was eluted with phosphate buffer containing 250 mM imidazole. _IN VITRO_
QUANTITATION OF CASPASE INHIBITION Caspases 1, 2, 3, 6, 7, 8, 9, 10, DRICE, DCP-1 and CED-3 were pre-activated for 10 min at 37°C in universal caspase citrate buffer (10 mM HEPES pH 7.0, 10%
sucrose, 0.1% CHAPS, 10 mM DTT, 100 mM NaCl, 1 mM EDTA, 0.65 M Na-Citrate). After the activation step, the caspase was incubated either with buffer alone, F-CED-91–251, AcP35-F or with
MaviP35-F for 1 h at 37°C. The appropriate fluorescent substrate was then added (100 _μ_M): Ac-WEHD-AFC for caspase 1; Ac-VDVAD-AFC for caspase 2; Ac-DEVD-AFC for caspases 3, 7, DRICE, DCP-1
and CED-3; Ac-VEID-AFC for caspase 6; Ac-LEHD-AFC for caspases 8, 9 and 10 and Ac-TQTD-AFC for DRONC (Enzo Life Sciences). Fluorescence (excitation 410 nm, emission 500 nm) was measured
every minute for 2 h. The maximal slope of each curve was calculated using Prism 5.0 and graphed. DETERMINATION OF INHIBITION CONSTANTS Caspases were pre-activated for 10 min at 37°C in the
following buffers: caspase 3: 100 mM HEPES pH 7.0, 10% PEG, 0.1% CHAPS, 10 mM DTT; DRICE: 50 mM HEPES pH 7.5, 10% sucrose, 0.1% CHAPS, 5 mM DTT, 100 mM NaCl, 1 mM EDTA; caspases 8 and 9: 10
mM HEPES pH 7.0, 10% sucrose, 0.1% CHAPS, 10 mM DTT, 100 mM NaCl, 0.1 mM EDTA, 0.65 M Na-Citrate; DRONC: 50 mM Tris pH 7.4, 100 mM NaCl, 0.65 M Na-Citrate. Subsequently, the caspase was
incubated with either AcP35-F or MaviP35-F in the appropriate activity buffer for 1 h at 37°C. Substrates were added at concentrations ranging from 0.001 to 1000 _μ_M. Substrates used were:
Ac-DEVD-AFC for caspase 3 and DRICE; Ac-LEHD-AFC for caspases 8 and 9 and Ac-VEID-AFC for DRONC (Enzo Life Sciences). Fluorescence (excitation 410 nm, emission 500 nm) was measured every
minute for 2 h. The slope of each curve was calculated using Prism 5.0. Inhibition constants were calculated by non-linear regression using Prism 5.0 software, using a competitive inhibition
model as described by these equations: _K_mObs=_K_m × (1+[_I_]/_K_i) and _Y_=_V_max × _X_/(_K_mObs+_X_), where [_I_] is the inhibitor concentration (_μ_M); _K_i is the inhibition constant
(_μ_M), _V_max is the maximum enzyme velocity (relative fluorescence units (RFU)/min), _K_m is the Michaelis–Menten constant (_μ_M), _X_ is the concentration of substrate (_μ_M) and _Y_ is
the change in fluorescence (RFU/min). ACCESSION CODES ACCESSIONS GENBANK/EMBL/DDBJ * AAF78504 * AAO12972 * AAO17287 * CAA71304 * NP_054165.1 * NP_703122 * YP_950833 ABBREVIATIONS * Mavi:
_Maruca vitrata_ * MNPV: multiple nucleopolyhedrovirus * Ac: _Autographa californica_ * Bm: _Bombyx mori_ * NPV: nucleopolyhedrovirus * Spli: _Spodoptera littoralis_ * TRAIL: TNF-related
apoptosis-inducing ligand * FBS: fetal bovine serum * MEF: mouse embryonic fibroblast * Xgal: 5-bromo-4-chloro-3-indolyl-_β_-D-galactopyranoside * CAT: chloramphenicol acetyl transferase *
RFU: relative fluorescence units REFERENCES * Ameisen JC . On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. _Cell Death Differ_ 2002; 9:
367–393. Article CAS PubMed Google Scholar * Best SM . Viral subversion of apoptotic enzymes: escape from death row. _Annu Rev Microbiol_ 2008; 62: 171–192. Article CAS PubMed PubMed
Central Google Scholar * Jabbour AM, Hawkins CJ . The p35 family of apoptosis inhibitors. _Curr Genomics_ 2004; 5: 215–229. Article CAS Google Scholar * Means JC, Penabaz T, Clem RJ .
Identification and functional characterization of AMVp33, a novel homolog of the baculovirus caspase inhibitor p35 found in Amsacta moorei entomopoxvirus. _Virology_ 2007; 358: 436–447.
Article CAS PubMed Google Scholar * Herniou EA, Olszewski JA, O’Reilly DR, Cory JS . Ancient coevolution of baculoviruses and their insect hosts. _J Virol_ 2004; 78: 3244–3251. Article
CAS PubMed PubMed Central Google Scholar * Clem RJ, Fechheimer M, Miller LK . Prevention of apoptosis by a baculovirus gene during infection of insect cells. _Science_ 1991; 254:
1388–1390. Article CAS PubMed Google Scholar * Xu G, Rich RL, Steegborn C, Min T, Huang Y, Myszka DG _et al_. Mutational analyses of the p35-caspase interaction. A bowstring kinetic
model of caspase inhibition by p35. _J Biol Chem_ 2003; 278: 5455–5461. Article CAS PubMed Google Scholar * Xu G, Cirilli M, Huang Y, Rich RL, Myszka DG, Wu H . Covalent inhibition
revealed by the crystal structure of the caspase-8/p35 complex. _Nature_ 2001; 410: 494–497. Article CAS PubMed Google Scholar * Riedl SJ, Renatus M, Snipas SJ, Salvesen GS .
Mechanism-based inactivation of caspases by the apoptotic suppressor p35. _Biochemistry_ 2001; 40: 13274–13280. Article CAS PubMed Google Scholar * Meier P, Silke J, Leevers SJ, Evan GI
. The _Drosophila_ caspase DRONC is regulated by DIAP1. _EMBO J_ 2000; 19: 598–611. Article CAS PubMed PubMed Central Google Scholar * Hawkins CJ, Yoo SJ, Petersen EP, Wang SL, Vernooy
SY, Hay BA . The _Drosophila_ caspase DRONC cleaves following glutamate or aspartate and is regulated by DIAP1, HID, and GRIM. _J Biol Chem_ 2000; 275: 27084–27093. CAS PubMed Google
Scholar * LaCount DJ, Hanson SF, Schneider CL, Friesen PD . Caspase inhibitor P35 and inhibitor of apoptosis Op-IAP block _in vivo_ proteolytic activation of an effector caspase at
different steps. _J Biol Chem_ 2000; 275: 15657–15664. Article CAS PubMed Google Scholar * Vier J, Furmann C, Hacker G . Baculovirus P35 protein does not inhibit caspase-9 in a cell-free
system of apoptosis. _Biochem Biophys Res Commun_ 2000; 276: 855–861. Article CAS PubMed Google Scholar * Morishima N, Okano K, Shibata T, Maeda S . Homologous p35 proteins of
baculoviruses show distinctive anti-apoptotic activities which correlate with the apoptosis-inducing activity of each virus. _FEBS Lett_ 1998; 427: 144–148. Article CAS PubMed Google
Scholar * Kamita SG, Majima K, Maeda S . Identification and characterization of the p35 gene of _Bombyx mori_ nuclear polyhedrosis virus that prevents virus-induced apoptosis. _J Virol_
1993; 67: 455–463. CAS PubMed PubMed Central Google Scholar * Gomi S, Zhou CE, Yih W, Majima K, Maeda S . Deletion analysis of four of eighteen late gene expression factor gene
homologues of the baculovirus, BmNPV. _Virology_ 1997; 230: 35–47. Article CAS PubMed Google Scholar * Pei Z, Reske G, Huang Q, Hammock BD, Qi Y, Chejanovsky N . Characterization of the
apoptosis suppressor protein P49 from the _Spodoptera littoralis_ nucleopolyhedrovirus. _J Biol Chem_ 2002; 277: 48677–48684. Article CAS PubMed Google Scholar * Zoog SJ, Schiller JJ,
Wetter JA, Chejanovsky N, Friesen PD . Baculovirus apoptotic suppressor P49 is a substrate inhibitor of initiator caspases resistant to P35 _in vivo_. _EMBO J_ 2002; 21: 5130–5140. Article
CAS PubMed PubMed Central Google Scholar * Lannan E, Vandergaast R, Friesen PD . Baculovirus caspase inhibitors P49 and P35 block virus-induced apoptosis downstream of effector caspase
DrICE activation in _Drosophila melanogaster_ cells. _J Virol_ 2007; 81: 9319–9330. Article CAS PubMed PubMed Central Google Scholar * Guy MP, Friesen PD . Reactive-site cleavage
residues confer target specificity to baculovirus P49, a dimeric member of the P35 family of caspase inhibitors. _J Virol_ 2008; 82: 7504–7514. Article CAS PubMed PubMed Central Google
Scholar * Jabbour AM, Ekert PG, Coulson EJ, Knight MJ, Ashley DM, Hawkins CJ . The p35 relative, p49, inhibits mammalian and _Drosophila_ caspases including DRONC and protects against
apoptosis. _Cell Death Differ_ 2002; 9: 1311–1320. Article CAS PubMed Google Scholar * Lee S-T, Srinivasan R, Lo Y-J, Talekar NS . Identification, characterization and bioassays of
_Maruca vitrata_ multiple nucleopolyhedrovirus (MaviNPV) against _Maruca vitrata_ (Lepidoptera, Pyralidae). _BioControl_ 2007; 52: 801–809. Article CAS Google Scholar * Chen YR, Wu CY,
Lee ST, Wu YJ, Lo CF, Tsai MF _et al_. Genomic and host range studies of _Maruca vitrata_ nucleopolyhedrovirus. _J Gen Virol_ 2008; 89: 2315–2330. Article CAS PubMed Google Scholar *
Fisher AJ, Cruz W, Zoog SJ, Schneider CL, Friesen PD . Crystal structure of baculovirus P35: role of a novel reactive site loop in apoptotic caspase inhibition. _EMBO J_ 1999; 18: 2031–2039.
Article CAS PubMed PubMed Central Google Scholar * Bertin J, Mendrysa SM, Lacount DJ, Gaur S, Krebs JF, Armstrong RC _et al_. Apoptotic suppression by baculovirus p35 involves cleavage
by and inhibition of a virus-induced CED-3/ICE-like protease. _J Virol_ 1996; 70: 6251–6259. CAS PubMed PubMed Central Google Scholar * Zhou Q, Krebs J, Snipas S, Price A, Alnemri E,
Tomaselli K _et al_. Interaction of the baculovirus anti-apoptotic protein p35 with caspases. Specificity, kinetics, and characterization of the caspase-p35 complex. _Biochemistry_ 1998; 37:
10757–10765. Article CAS PubMed Google Scholar * Dorstyn L, Read S, Cakouros D, Huh JR, Hay BA, Kumar S . The role of cytochrome _c_ in caspase activation in _Drosophila melanogaster_
cells. _J Cell Biol_ 2002; 156: 1089–1098. Article CAS PubMed PubMed Central Google Scholar * Corpet F . Multiple sequence alignment with hierarchical clustering. _Nucleic Acids Res_
1988; 16: 10881–10890. Article CAS PubMed PubMed Central Google Scholar * Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ . Jalview Version 2 – a multiple sequence alignment
editor and analysis workbench. _Bioinformatics_ 2009; 25: 1189–1191. Article CAS PubMed PubMed Central Google Scholar * Kelley LA, Sternberg MJ . Protein structure prediction on the
Web: a case study using the Phyre server. _Nat Protoc_ 2009; 4: 363–371. Article CAS PubMed Google Scholar * Hawkins CJ, Wang SL, Hay BA . Monitoring activity of caspases and their
regulators in yeast _Saccharomyces cerevisiae_. _Methods Enzymol_ 2000; 322: 162–174. Article CAS PubMed Google Scholar * Hawkins CJ, Wang SL, Hay BA . A cloning method to identify
caspases and their regulators in yeast: identification of _Drosophila_ IAP1 as an inhibitor of the _Drosophila_ caspase DCP-1. _Proc Natl Acad Sci_ 1999; 96: 2885–2890. Article CAS PubMed
PubMed Central Google Scholar * Hawkins CJ, Silke J, Verhagen AM, Foster R, Ekert PG, Ashley DM . Analysis of candidate antagonists of IAP-mediated caspase inhibition using yeast
reconstituted with the mammalian Apaf-1-activated apoptosis mechanism. _Apoptosis_ 2001; 6: 331–338. Article CAS PubMed Google Scholar * Jabbour AM, Ho PK, Puryer MA, Ashley DM, Ekert
PG, Hawkins CJ . The _Caenorhabditis elegans_ CED-9 protein does not directly inhibit the caspase CED-3, _in vitro_ nor in yeast. _Cell Death Differ_ 2004; 11: 1309–1316. Article CAS
PubMed Google Scholar * Clem RJ, Miller LK . Control of programmed cell death by the baculovirus genes p35 and iap. _Mol Cell Biol_ 1994; 14: 5212–5222. Article CAS PubMed PubMed
Central Google Scholar * Hawkins CJ, Uren AG, Hacker G, Medcalf RL, Vaux DL . Inhibition of interleukin 1-beta-converting enzyme-mediated apoptosis of mammalian cells by baculovirus IAP.
_Proc Natl Acad Sci USA_ 1996; 93: 13786–13790. Article CAS PubMed PubMed Central Google Scholar * Hay BA, Wolff T, Rubin GM . Expression of baculovirus P35 prevents cell death in
_Drosophila_. _Development_ 1994; 120: 2121–2129. CAS PubMed Google Scholar * Clarke TE, Clem RJ . Lack of involvement of haemocytes in the establishment and spread of infection in
_Spodoptera frugiperda_ larvae infected with the baculovirus _Autographa californica_ M nucleopolyhedrovirus by intrahaemocoelic injection. _J Gen Virol_ 2002; 83: 1565–1572. Article CAS
PubMed Google Scholar * Huang N, Wu W, Yang K, Passarelli AL, Rohrmann GF, Clem RJ . Baculovirus infection induces a DNA damage response that is required for efficient viral replication.
_J Virol_ 2011; 85: 12547–12556. Article CAS PubMed PubMed Central Google Scholar * Wang H, Blair CD, Olson KE, Clem RJ . Effects of inducing or inhibiting apoptosis on Sindbis virus
replication in mosquito cells. _J Gen Virol_ 2008; 89: 2651–2661. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Chung-Hsiung Wang for providing the
plasmid bearing the _MaviP35_ gene, Sam Le Fort and David Vaux for the pAct5c-eGFP plasmid, Gary Hime for the Kc167 cells and Anissa Jabbour and Paul Ekert for the MEF cells. This work was
funded by the National Health and Medical Research Council project grant to CJH and RJC (#602525), an Australian Research Council Future Fellowship to CJH (#FT0991464), scholarships to ILB
and MML from the La Trobe University and a scholarship to ILB from the Cooperative Research Centre for Biomarker Translation. AUTHOR INFORMATION Author notes * M M Green, S Civciristov and D
Pantaki-Eimany: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Biochemistry, La Trobe Institute for Molecular Science, La Trobe University,
Bundoora, Victoria, Australia, I L Brand, M M Green, S Civciristov, D Pantaki-Eimany, C George & C J Hawkins * Division of Biology, Kansas State University, Manhattan, KS, USA T R Gort,
N Huang & R J Clem * Children's Cancer Centre, Murdoch Children's Research Institute, Royal Children's Hospital, Parkville, Australia C J Hawkins Authors * I L Brand View
author publications You can also search for this author inPubMed Google Scholar * M M Green View author publications You can also search for this author inPubMed Google Scholar * S
Civciristov View author publications You can also search for this author inPubMed Google Scholar * D Pantaki-Eimany View author publications You can also search for this author inPubMed
Google Scholar * C George View author publications You can also search for this author inPubMed Google Scholar * T R Gort View author publications You can also search for this author
inPubMed Google Scholar * N Huang View author publications You can also search for this author inPubMed Google Scholar * R J Clem View author publications You can also search for this author
inPubMed Google Scholar * C J Hawkins View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to C J Hawkins. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Edited by P Salomoni RIGHTS AND PERMISSIONS This work is licensed under the Creative
Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Brand, I., Green, M., Civciristov, S. _et al._ Functional and biochemical characterization of the baculovirus caspase inhibitor MaviP35. _Cell Death Dis_ 2,
e242 (2011). https://doi.org/10.1038/cddis.2011.127 Download citation * Received: 01 November 2011 * Accepted: 14 November 2011 * Published: 15 December 2011 * Issue Date: December 2011 *
DOI: https://doi.org/10.1038/cddis.2011.127 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * caspase * P35 * baculovirus * apoptosis * infection *
virus