Pathogen response-like recruitment and activation of neutrophils by sterile immunogenic dying cells drives neutrophil-mediated residual cell killing
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Innate immune sensing of dying cells is modulated by several signals. Inflammatory chemokines-guided early recruitment, and pathogen-associated molecular patterns-triggered
activation, of major anti-pathogenic innate immune cells like neutrophils distinguishes pathogen-infected stressed/dying cells from sterile dying cells. However, whether certain sterile
dying cells stimulate innate immunity by partially mimicking pathogen response-like recruitment/activation of neutrophils remains poorly understood. We reveal that sterile immunogenic dying
cancer cells trigger (a cell autonomous) pathogen response-like chemokine (PARC) signature, hallmarked by co-release of CXCL1, CCL2 and CXCL10 (similar to cells infected with bacteria or
viruses). This PARC signature recruits preferentially neutrophils as first innate immune responders _in vivo_ (in a cross-species, evolutionarily conserved manner; in mice and zebrafish).
Furthermore, key danger signals emanating from these dying cells, that is, surface calreticulin, ATP and nucleic acids stimulate phagocytosis, purinergic receptors and toll-like receptors
(TLR) i.e. TLR7/8/9-MyD88 signaling on neutrophil level, respectively. Engagement of purinergic receptors and TLR7/8/9-MyD88 signaling evokes neutrophil activation, which culminates into
H2O2 and NO-driven respiratory burst-mediated killing of viable residual cancer cells. Thus sterile immunogenic dying cells perform 'altered-self mimicry' in certain contexts to
exploit neutrophils for phagocytic targeting of dead/dying cancer cells and cytotoxic targeting of residual cancer cells. SIMILAR CONTENT BEING VIEWED BY OTHERS STRATEGIES OF NEUTROPHIL
DIVERSIFICATION Article 23 March 2023 CLEARANCE OF APOPTOTIC CELLS BY NEUTROPHILS IN INFLAMMATION AND CANCER Article Open access 13 January 2024 THE DIVERSE ROLES OF NEUTROPHILS FROM
PROTECTION TO PATHOGENESIS Article 20 November 2024 MAIN Sensing of dying/dead cells by innate immune cells forms the core of tissue homeostasis and various diseases.1 Thus, the molecular
entities governing this interface are of great interest. Over the last decade, three main innate immune-modulatory profiles of sterile cell death (i.e., cell death induced by non-microbial
stimuli) have been demarcated, that is, tolerogenic apoptosis, necrosis and damage-associated molecular patterns (DAMPs)-linked apoptosis (or immunogenic apoptosis).2, 3 In general,
modulation of the vertebrate innate immunity is explained by two cardinal models, that is, the 'self/non-self model'4 and the 'danger model'.5 Interestingly, these models
contradict on cell death immunology. The self/non-self model postulates the activation of innate immunity only by entities of 'non-self' (e.g., pathogens) or
'altered-self' (e.g., pathogen-infected host cell) origins, possessing pathogen-associated molecular patterns (PAMPs) sensed via pattern recognition receptors (PRRs).4 This model
maintains that PRR ligands cannot be derived from endogenous sources.6 Conversely, the 'danger model' postulates that non-physiological, sterile, cell death can activate the innate
immune system by releasing endogenous DAMPs, a subset of which are potent danger signals and agonists of PRRs like toll-like receptors (TLRs).5 Research from various labs7, 8 including
ours3, 9 has credibly validated the danger model and shown that DAMPs or danger signals emanating from dying (cancer) cells indeed accentuate sensing of dying cells by the innate immune
cells. Such liberation of DAMPs can either be achieved in an unregulated fashion by (accidental/regulated) necrosis7, 10 or in a spatiotemporally regulated fashion through immunogenic
apoptosis.8 Thus, according to the current conceptualizations, although the self/non-self model explains the tolerogenic apoptosis profile yet the danger model alone explains the
immunostimulatory profiles of necrosis and immunogenic apoptosis.3, 4, 5 However, the analogy between PAMPs and DAMPs has ignited a long-standing unresolved question, that is, can certain
dying cells partially mimic behavior of a pathogen-infected cell? If this would be the case this ‘altered-self mimicry’ could rectify why certain forms of sterile cell death drive innate
immune stimulation and reconcile the two models in one paradigm. At the site of pathogenic invasion (typically peri-/intra-epithelial milieus),11 in parallel with local phagocytic activity
by sentinel cells, one of the first inflammatory processes triggered by an altered self cell to limit further damage entails production of specific inflammatory (or dual function) chemokines
to recruit major anti-pathogenic innate immune cells, for example, neutrophils.11, 12, 13 Such chemokine-based recruitment eventually paves the way for phagocytosis and direct elimination
of (residual) pathogens by innate immune cells.12, 14 To this end, we deemed it necessary to probe whether sterile dying cells, and in particular those undergoing DAMP-linked cell demise,
can recruit (via specific chemokines) and activate innate immune cells in a pathogen response-like fashion culminating into cytotoxicity against residual viable cells. RESULTS IMMUNOGENIC
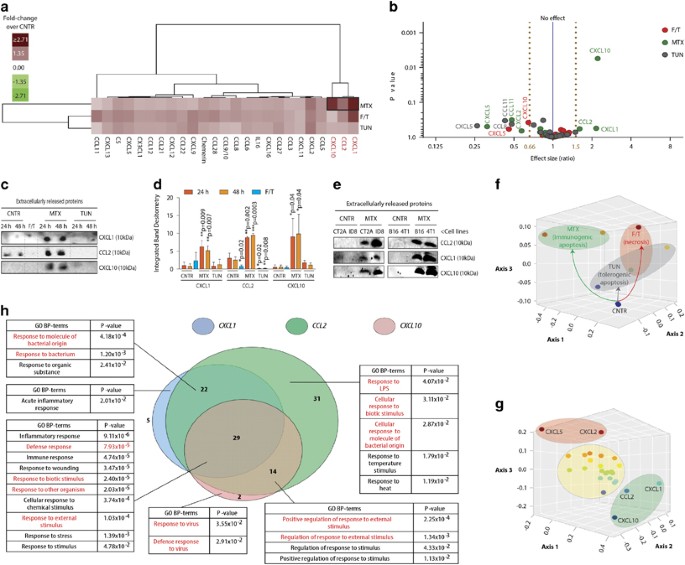
APOPTOSIS, BUT NOT ACCIDENTAL NECROSIS OR TOLEROGENIC APOPTOSIS, CAUSES CO-RELEASE OF CXCL1, CCL2 AND CXCL10 CHEMOKINES Initially, we examined the chemokines released during accidental
necrosis, tolerogenic apoptosis or immunogenic apoptosis. We assessed the release of 25 major murine chemokines (encompassing key inflammatory/homeostatic/dual-function chemokines;13
Supplementary Figure S1A) in the cell-free-conditioned medium (CM) derived from the low-immunogenic LLC lung epithelial carcinoma cells undergoing tolerogenic apoptosis (induced by
tunicamycin (TUN))15, 16 or immunogenic apoptosis (induced by mitoxantrone (MTX))15, 16, 17 and compared them to accidental necrosis (induced by freeze/thawing or F/T).15, 17 Of note, TUN,
F/T and MTX are _bona fide_ inducers of these respective cell death immune profiles as published by us15, 17 and others.16, 18 At similar cell death-inducing doses, (~70% cell death;
Supplementary Figure S1B) primarily CM derived from MTX-treated cells (but not F/T or TUN) associated with increased co-release of specific chemokines, that is, CXCL1, CCL2 and CXCL10
(Figures 1a and b). A volcano plot based on the same data confirmed that only MTX caused >1.5-fold increase in these chemokines’ release (Figure 1b). This was further substantiated by
direct immunoblotting of CM derived from respective dying cells (Figures 1c and d). Beyond MTX, only F/T caused some variable, albeit non-significant, increase in CXCL1/CCL2 release (Figures
1a, b and d). Importantly, other known immunogenic apoptosis inducers (radiotherapy>photodynamic therapy/PDT), but not tolerogenic apoptosis inducer (cisplatin),3 also caused increased
co-release of CXCL1, CCL2 and CXCL10 (Supplementary Figure S1C). The co-release of CXCL1, CCL2 and CXCL10 following MTX treatment was also evident in other cell lines/cell types, for
example, CT2A, ID8, B16 and 4T1 cells (Figure 1e) suggesting a broad cellular association between this signature and immunogenic apoptosis. In general, MTX-induced immunogenic apoptosis
exhibited the most divergent chemokine profile, evident from its spatially distinct, non-overlapping, positioning (relative to F/T-treated/TUN-treated/untreated cells) within a non-metric
multidimensional scaling 3D environment (Figure 1f). Similarly, in this same statistical environment, CXCL1, CCL2 and CXCL10 formed a spatially distinct cluster – signifying their tendency
to be co-released/co-regulated in this context (Figure 1g). CO-RELEASE OF CXCL1, CCL2 AND CXCL10 REPRESENTS A PATHOGEN RESPONSE-LIKE CHEMOKINE SIGNATURE Next, we analyzed whether the
CXCL1-CCL2-CXCL10 cluster represents a pathogen response-like chemokine (PARC) signature. Using Gene Ontology (GO) bioinformatics analysis we observed that these three chemokines,
statistically significantly, enumerated biological process (BP) terms (highlighted in red, Figure 1h) associated with responses to pathogens; especially bacteria, molecules of bacterial
origin (e.g., lipopolysaccharide (LPS)) or viruses (Figure 1h). To further test the validity of CXCL1-CCL2-CXCL10 cluster as a _bona fide_ PARC signature, we first performed a comprehensive
meta-analysis of Gene Expression Omnibus (GEO) data sets consisting of experiments involving mammalian cells/tissues treated with various pathogens (or pathogen-derived products) including
bacteria, viruses and protozoan parasites (Figure 2a). Indeed, many pathogens or pathogen-origin products upregulated CXCL1-/CCL2-/CXCL10-coding genes (Figure 2a) wherein these exhibited
significant co-upregulation (Figure 2b). Interestingly, CXCL1/CCL2/CXCL10’s tendency toward co-upregulation was highest in response to bacteria/bacterial products (Figure 2c) and viruses
(Figure 2d), but least to protozoan parasites (Figure 2e). Certain bacterial-origin products (e.g., LPS) or known oncolytic viruses14 also upregulated CXCL1/CCL2/CXCL10-coding genes
(highlighted in red, Figure 2a). Henceforth, we experimentally challenged LLC cells with bacterial-origin products (i.e., LPS and flagellin) or well-established oncolytic viruses (reovirus,
parvovirus and Newcastle disease virus (NDV)14, 19 (Supplementary Figure S2). Remarkably, these bacterial products (Figure 2f) and oncolytic viruses (Figure 2g) caused increased co-release
of CXCL1/CCL2/CXCL10 in a temporally defined manner (although bacterial products sustained the chemokine co-release better, thereby substantiating the GEO meta-analysis; Figures 2b–e).
Finally pooled analysis of the results in Figure 1c/Figures 2f–g/Supplementary Figure S1C revealed the tendency of immunogenic apoptosis inducers (MTX/radiotherapy/PDT) to cluster together
with bacterial-origin products and oncolytic viruses in terms of increased co-release of CXCL1/CCL2/CXCL10; whereas necrosis (F/T) and tolerogenic apoptosis (TUN/cisplatin) clustered apart
(Figure 2h). These data collectively indicate that immunogenic apoptosis, but not accidental necrosis or tolerogenic apoptosis, associates with a 'putative' PARC signature.
IMMUNOGENIC APOPTOSIS SELECTIVELY RECRUITS NEUTROPHILS AS FIRST INNATE IMMUNE RESPONDERS Next, we tested whether immunogenic apoptotic cells can attract innate immune cells in a manner
reminiscent of an altered self cell, for example, rapid, early recruitment of neutrophils.11, 12, 16 We used a model (Figure 3a), in which the necrotic or (tolerogenic/immunogenic) apoptotic
LLC cells were injected intra-dermally into ear pinna of syngeneic mice (Supplementary Figure S3A); followed by estimation of early chemotactic recruitment of various (CD45+) leukocytes
(Figure 3b) like (CD11b+Ly6G+) neutrophils (Figure 3c), (CD11b+F4/80+) macrophages (Figure 3d), (CD11c+) monocytes/dendritic cells (DCs) (Figure 3e), (CD3+) T cells (Figure 3g) and (B220+) B
cells/plasmacytoid DCs (pDCs)/lymphokine-activated cells (Figure 3f). Immunogenic apoptosis triggered increased early recruitment of leukocytes in comparison to PBS/CNTR (Figure 3b).
Strikingly, only immunogenic apoptosis but not necrosis/tolerogenic apoptosis, caused a significantly sustained, rapid, recruitment of neutrophils (Figure 3c), which was preferred over other
major leukocytic populations (Figures 3d–g). This is in contrast to previous reports where monocytes/macrophages, rather than neutrophils, have been demonstrated to preferentially respond
to apoptosis.20 Although macrophages/monocytes/DCs did not exhibit early recruitment, they can still be (co-)involved in local sentinel immunological activity.1 We further confirmed the
increased recruitment of neutrophils in ear pinna injected with MTX-induced dying cells through immunohistochemistry (Figures 3h and i) and by documenting typical polymorphonuclear
neutrophils (i.e., PMNs with ⩾3-lobed nucleus) (Figure 3j). IMMUNOGENIC APOPTOSIS-TRIGGERED NEUTROPHIL RECRUITMENT DEPENDS ON PATHOGEN RESPONSE-LIKE CHEMOKINE SIGNATURE Neutrophils are
typically the first innate immune responders to infections12 and, under PAMPs’ influence they perform crucial functions like phagocytic clearance and direct pathogen elimination.12, 14 Our
results raised precedence to investigate whether the PARC signature is responsible for this neutrophil recruitment. GO bioinformatics analysis indicated an association between
CXCL1-CCL2-CXCL10 and ‘neutrophil chemotaxis’ (Supplementary Figure S3B). Also an unbiased correlation analysis (involving chemokines exhibiting >1.2-fold increase in release by at least
one cell death pathway, Figure 1a) revealed that release of CXCL1/CCL2/CXCL10 was maximally positively correlated (⩾0.80) with the levels of neutrophil chemotaxis (Figure 3k), across both
time points. To establish a cause–effect relationship, the dead/dying cells were deprived of the PARC signature via specific blocking antibodies before injection. This simultaneous blockade
of CXCL1-CCL2-CXCL10 markedly decreased neutrophils' recruitment toward MTX-treated cells (Figure 3l). Above observations were not specific for LLC cells; as MTX-treated B16 cells also
significantly chemo-attracted neutrophils and simultaneous blockade of CXCL1-CCL2-CXCL10 significantly reduced this neutrophil recruitment (Supplementary Figure S3C). To analyze the
neutrophil-attracting properties of individual chemokines, we expressed in LLC cells, shRNAs targeting CXCL1 or CCL2 or CXCL10, thereby severely suppressing their release following MTX
treatment (Supplementary Figure S4). This individual abrogation of each chemokine significantly affected the _in vivo_ recruitment of neutrophils by MTX-treated cells in a hierarchical,
chemokine-dependent fashion (CXCL1≈CXCL10»CCL2) (Figure 3m). Thus PARC signature sustains a dominant neutrophil-attracting phenotype, which allows immunogenic apoptosis to recruit
neutrophils as first innate immune responders. To further validate the CXCL1/CCL2/CXCL10 co-regulation as a PARC signature, we performed a ‘reverse-enumeration’ bioinformatics analysis
wherein murine chemokines were selected (from the entire ‘chemokinome’) in an unbiased manner using the major pathogen response and neutrophil chemotaxis related GO BP terms (highlighted in
red, Figure 4a). This analysis strongly enumerated for CXCL1/CCL2/CXCL10 (Figure 4b) and some of their closely homologous chemokines (Figures 4b and c) within the uppermost quartile (Q3).
This further positions CXCL1-CCL2-CXCL10 as constituting a dominant, albeit non-exclusive, neutrophil-recruiting PARC signature.11, 13 PATHOGEN RESPONSE-LIKE CHEMOKINE SIGNATURE-DRIVEN
NEUTROPHIL RECRUITMENT BY IMMUNOGENIC APOPTOSIS IS EVOLUTIONARILY CONSERVED IN A CROSS-SPECIES MANNER In principle, the PARC signature-based neutrophil recruitment should be evolutionarily
conserved across vertebrates since chemokines evolved ~650 million years ago in fish – a quintessential feature of vertebrate immunity21 (for instance zebrafish, but not fruitfly/roundworms,
possess homologs of several murine chemokines21 including _Ccl2/Cxcl10_, Supplementary Figure S5A). Similarly, as per self/non-self model,4 our results obtained in a ‘self set-up’ (murine
dying cells in syngeneic mice) are expected to maintain at least some of their specificity in a ‘non-self setup’ (in a cross-species manner, e.g., murine dying cells in zebrafish).
Henceforth, we injected F/T (negative control) or MTX-treated LLC cells in the yolk sac of zebrafish larvae (Supplementary Figures S5B and C) transgenic for GFP+neutrophils (_mpx:GFP_) and
mCherry+macrophages (_fms_:_nfsB.mCherry_)22 (larval zebrafish, at this developmental stage, have a simple innate immune system dominated by these two cell types)22, 23 (Supplementary Figure
S6A). Similar to the ‘self set-up’, MTX-induced dying cells (but not F/T-induced) specifically recruited zebrafish neutrophils (Supplementary Figures S6B–D). Conversely, while MTX-treated
cells did not chemoattract macrophages yet F/T-treated cells did (Supplementary Figures S6B and C). Next, we injected MTX-treated cells deprived of CXCL1-CCL2-CXCL10 via antibody-based
blockade into the zebrafish larvae's yolk sac. This markedly decreased neutrophils' recruitment into the yolk sac (Supplementary Figures S7A and B). We also injected different
zebrafish larvae with MTX-treated cells with or without individual shRNAs against CXCL1 or CCL2, or CXCL10 (Supplementary Figure S4). Interestingly, CXCL10 and CCL2 depletion (CXCL10≈CCL2)
but not CXCL1 depletion, affected the _in vivo_ recruitment of neutrophils (Supplementary Figures S7C and D). These results are partially dissimilar to mice setup (CXCL1≈CXCL10»CCL2; Figure
3m) but in line with the bioinformatics analysis showing the presence of _Ccl2/Cxcl10_ homologs but not _Cxcl1_ homolog in zebrafish (Supplementary Figure S5A). These results demonstrate the
ability of immunogenic apoptosis-associated PARC signature to recruit neutrophils in a cross-species (evolutionarily conserved) fashion. IMMUNOGENIC APOPTOSIS TRIGGERS NEUTROPHIL-DRIVEN
PHAGOCYTOSIS AND PRO-INFLAMMATORY STIMULATION Next, we investigated the neutrophils-dying cells interaction in _ex vivo_ co-cultures. Phagocytosis analysis was done with LLC cells stained
through pH-sensitive dye (pHrodo) that emits heightened fluorescence upon phagosome contact (pH≈5), thereby distinguishing engulfed from un-engulfed cells. A fraction of MTX-treated LLC
cells underwent significant phagocytic clearance by neutrophils (Figures 5a and b). MTX-treated cells surface exposed (ecto-) two important 'eat me' signals, that is,
phosphatidylserine (ecto-PtdSer) (Supplementary Figure S1B) and calreticulin (ecto-CRT), but not heat-shock protein90 (HSP90) (Figure 5c).24 Disruption of these pro-phagocytic signals
(PtdSer by annexin-V and CRT by antibody) significantly decreased engulfment by neutrophils (Figures 5a and b). Neutrophils tended to prefer ecto-CRT as an 'eat me' signal over
PtdSer (Figure 5b). Moreover, neutrophils interacting with MTX-treated cells underwent strong phenotypic (CD86high/MHC-IIhigh) and functional (IL6highIL1_β_highIL10low) maturation (Figures
5d and e). Neutrophils interacting with F/T-treated cells (negative control) remained largely immature (Figures 5d and e). Importantly MTX-treated LLC (Figure 5f) or B16 cells (Supplementary
Figure S8A) caused increased accumulation of CD86high/MHC-IIhighneutrophils _in vivo_ thus confirming the physiological relevance of this neutrophil-based sensing. Disruption of neutrophil
recruitment through shRNA-based depletion of CXCL1 or CCL2, or CXCL10 also resulted in proportionately reduced accumulation of CD86high/MHC-IIhigh neutrophils _in vivo_ (Figure 5f)
(CXCL1≈CXCL10»CCL2). In fact there was a strong positive correlation between neutrophil recruitment and accumulation of activated neutrophils _in vivo_ (Supplementary Figure S8B) –
indicating that disruption of ‘upstream’ neutrophil recruitment also compromises ‘downstream’ neutrophil maturation/sensing. IMMUNOGENIC APOPTOSIS STIMULATES NEUTROPHILS BY ENGAGING
EXTRACELLULAR NUCLEIC ACIDS–MYD88-TLR7/8/9 AND EXTRACELLULAR ATP-PURINERGIC RECEPTOR SIGNALING AXES PARC signature did not seem to directly modulate neutrophil-based sensing, as blocking it
didn’t negatively affect neutrophil activation (Supplementary Figure S8C), thereby implying presence of other immunostimulatory factors. Immunogenic apoptotic cells emit DAMPs-like ATP
(agonist of purinergic-2/P2 receptors/P2Rs), high-mobility group box 1 (HMGB1) protein or HSPs-70/90 (agonists of TLRs myeloid differentiation primary response 88/MyD88 pathway) or
annexin-A1 (AnxA1),3, 7 known to operate at the dying cell-DC/macrophage interface.3, 24 However, not much clarity exists on dying cell-derived neutrophil-activating factors/danger signals.
In line with the previous studies,2, 17, 25 in response to MTX, immunogenic apoptotic LLCs released ATP (Figure 6a), HMGB1 and AnxA1 (but not HSP70/90) (Figure 6b). Interestingly, blocking
strategies blunting these DAMP’s presence/function revealed that only ATP degradation (via apyrase/Apy enzyme), but not HMGB1-blockade (Figure 6c) abrogated neutrophil stimulation.
Conversely, AnxA1 acted as a partial ‘anti-inflammatory DAMP’ as blocking it increased neutrophil's CD86 levels (Figure 6c). Next, we blocked the three most important neutrophil
PRRs/receptor–adaptor systems,12, 26 that is, formyl peptide receptor 1 (FPR1), P2Rs family and MyD88 (TIR adaptor protein relevant for most TLRs, except TLR3)7 in neutrophil–LLC co-cultures
(Figure 6d). Blocking P2Rs or MyD88 activity (but not FPR1) compromised neutrophil maturation (Figure 6e). However, blocking MyD88 exerted higher negative effect on neutrophil stimulation
(CD86↓/MHC-II↓) than P2Rs inhibition (MHC-II↓) (Figure 6e). Furthermore, neutrophils derived from _Myd88_−/−mice phenocopied this dominant effect of MyD88 peptide antagonist, on neutrophil
stimulation (Figure 6f), thereby inferring an important role for TLR signaling. While we delineated the P2Rs agonist (ATP), the TLR agonist remained obscure. During pathogenic infections
apart from protein PAMPs (lipoproteins/flagellin/viral proteins), cell wall-derived (LPS/lipoteichoic acid) or nucleotidic-PAMPs (viral/bacterial nucleic acids) also drive TLR signaling.26,
27 As the current setup exhibited features of pathogen response-like signaling, we wondered whether MTX-treated cells released analogous nucleotidic DAMPs.28 Analysis of cell-free CM from
MTX-treated cells revealed significant enrichment of nucleic acids (Figure 6g) including double-stranded DNA (Figure 6h). The release of nucleic acids was a general consequence of cell death
but their overall quantities were inducer-dependent (Supplementary Figures S9A and B). Therefore, we degraded the nucleic acids released by MTX-treated cells (via DNase/RNase enzymes) and
observed remarkable reduction in neutrophil maturation (similar to _Myd88_−/−neutrophils) (Figure 6i). Nucleic acids-based danger signals tend to bind/signal through various cognate TLRs,
for example, TLR3, TLR7/8 and TLR9.26, 27 Hence we blocked these through selective pharmacological inhibition (CU-CPT4a against TLR3) or specific oligodinucleotide (ODN) antagonists of
TLR7/8 (ODN2087) or TLR9 (ODN2088).29 Inhibition of TLR7/8/9 (wherein TLR9>TLR7/8), but not TLR3, ablated neutrophil stimulation (Figure 6j). Interestingly, a GO bioinformatics analysis
of TLR7/8/9-MyD88 axis significantly enumerated for GO terms relevant for pathogen response-like signaling (especially anti-viral response) (Supplementary Figure S9C). These effects of
ATP-P2Rs and nucleic acids–TLR7/8/9-MyD88 axes were not confined to neutrophil phenotypic maturation. Blockade of these signaling axes also drastically shifted the (extracellular) cytokines
profile of neutrophils interacting with MTX-treated cells, from pro-inflammatory (IL6highIL1_β_highIL10low) to anti-inflammatory (IL6lowIL1_β_lowIL10high) (Figure 6k). Here, IL1_β_ was
co-induced by both axes, whereas IL6 was induced (and IL10 was suppressed) only by nucleic acids–TLR7/8/9-MyD88 axes (Figure 6k). Blockers of FPR1/extracellular HMGB1/extracellular
AnxA1/TLR3 failed to affect the cytokines patterns (Figure 6k). NEUTROPHILS STIMULATED BY IMMUNOGENIC APOPTOSIS EXERT CYTOTOXICITY AGAINST RESIDUAL LIVE CANCER CELLS PAMPs-activated
neutrophils typically target/eliminate residual pathogens to facilitate resolution-of-inflammation.12 Hence, we determined whether the DAMPs-activated neutrophils exerted cytotoxicity
against residual cancer cells that managed to survive MTX-induced cell death (Supplementary Figure S1B). Indeed, MTX-treated LLC cells (CD11b−Ly6G−) co-cultured with neutrophils
(CD11b+Ly6G+) underwent significantly more cell death (~90%) than MTX-treated LLC cells alone (~70%) (Figure 6l). Remarkably, blockade of ATP-P2Rs and nucleic acids–TLR7/8/9-MyD88 axes
reduced the neutrophil-dependent cell death of residual cells that survived the MTX insult (Figure 6m). Thus, neutrophils interacting with immunogenic apoptotic cells gain a pro-inflammatory
profile, culminating into neutrophil-dependent cytotoxicity against residual cancer cells. NEUTROPHILS-MEDIATED CYTOTOXICITY AGAINST RESIDUAL LIVE CELLS IS DRIVEN BY RESPIRATORY BURST
Normally neutrophils exert cytotoxic effects through extrinsic molecular mechanisms (e.g., FasL/perforins-based, against eukaryotic cells) or respiratory burst (e.g., H2O2-/NO-based,
typically against pathogens).12, 26, 30, 31 Neutrophils interacting with MTX-induced dying cells did not appreciably upregulate FasL/perforins (Supplementary Figures S10A and B); however,
they significantly released H2O2 and NO (Figure 6n). The ATP-P2Rs and nucleic acids–TLR7/8/9-MyD88 axes also tightly regulated neutrophil-driven respiratory burst (NO production was
co-induced by both, whereas H2O2 production was driven by ATP-P2Rs axis) (Figure 6n). Next, we blocked the respiratory burst through various agents,30, 31 for example, apocynin (blocks NADPH
oxidase complex-based H2O2 production), catalase (degrades H2O2) and L-NMMA (blocks NOS-based NO production) (Supplementary Figures S11A–C). Curiously, blockade of either H2O2 or NO
production inhibited neutrophil-dependent residual cell killing (Figure 6o) suggesting combinatorial effect of these two oxidative species. Furthermore, the pan-caspase inhibitor, zVAD,
significantly reduced neutrophil-dependent residual cell killing (Figure 6o) thereby indicating that H2O2 and/or NO induce apoptosis in residual cancer cells. DISCUSSION Collectively, the
current study demonstrates elicitation of pathogen response-like innate immune signaling or 'altered-self mimicry' during sterile immunogenic apoptosis on multiple levels (Figure
7), that is, (i) dying cell autonomous co-release of CXCL1/CCL2/CXCL10 constituting a PARC signature, observed against bacteria/bacterial products and viruses (bacteria>viruses) rather
than protozoan parasites, (ii) neutrophils as first innate immune responders to these dying cells _in vivo_, (iii) cross-species evolutionary conservation of PARC signature-based neutrophil
recruitment (mice _versus_ zebrafish), (iv) regulation of neutrophil activation and neutrophil-dependent cytotoxicity through ATP-P2Rs and nucleic acids–TLR7/8/9-MyD88 axes and (v) a
pathogen response-like elimination of residual cells via (H2O2-/NO-driven) respiratory burst. Our results are partially supported by another study where doxorubicin-treated immunogenic dying
cells exhibited autonomous ‘dsRNA virus mimicry’, that is, self-dsRNA:TLR3 interaction causing production of type I interferons.25 However, our study reveals a much broader
'altered-self mimicry' phenotype consisting of not only a dying cell autonomous 'mimicry', that is, the PARC signature that at least partially resembles responses to both
bacteria/bacterial products and viruses, but also neutrophil-level 'mimicry' involving pathogen response-like recruitment/activation and residual cell-targeting activity. In
future it would be interesting to uncover the cell autonomous pathways governing the co-release of CCL2, CXCL1 and CXCL10 from immunogenic dying cells. The limited accountability for
neutrophil modulation by dying cells perhaps stems from the particular emphasis on mechanisms of antigen recovery and presentation from cell corpses,5 a process better executed by DCs and
macrophages.3, 24 Thus, chemokines or 'find me’ signals, ‘eat me’ signals and DAMPs operating at the neutrophil-dying cell interface and influencing neutrophil-dependent cytotoxicity
are poorly understood. Here we provide several intriguing insights into how neutrophils sense dying/dead cells by uncovering hitherto unclear mechanisms collectively coordinated through
chemokines, ecto-CRT, nucleic acids and ATP. Also, previously most studies have focused on neutrophil-dependent cytotoxicity against a subset of untreated cancer cells,30, 31 whereas
mechanisms of targeting residual cancer cells that have survived a drug-induced cytotoxic insult were seldom described. Henceforth, co-ordination of ATP-P2Rs and nucleic acids–TLR7/8/9-MyD88
axes underlying neutrophil-dependent cytotoxicity against residual cells that survived MTX insult is an interesting finding. Similarly, positioning of ecto-CRT as neutrophils’ preferred
‘eat me’ signal is captivating. The discovery of (cross-species) evolutionarily conserved ability of murine CCL2/CXCL10 to chemoattract zebrafish neutrophils is also intriguing. Although
existence of CCL2-like23 and CXCL10-like32 chemokinetic activity in zebrafish has been reported previously, the ability of these chemokines to attract neutrophils was seldom described. Thus
our results provide interesting insights into the versatility of zebrafish chemokine biology and redundancy among vertebrate chemokine activity. Moreover, the dominant neutrophil-attracting
effect of CXCL10 (but not CXCL1) across both mice and zebrafish is noteworthy. The existence of PARC signature in different cell types (and induction by various immunogenic apoptotic
stimuli), indicates its broad relevance as neutrophil recruitment signal. However, it is not clear whether PARC signature is restricted to immunogenic sub-form of only apoptosis or also of
other regulated cell death pathways. Moreover, considerable redundancy in chemokines' functions12, 21 means that we cannot exclude the possibility of chemokines within the PARC
signature being replaced or co-supported by other (homologous) chemokines with overlapping neutrophil-recruiting potential in a context-dependent fashion (Figure 4). Last but not the least,
the tissue milieu where these dying cells were injected may also be an influencing factor in the final outcome of the innate immune responses.11 As such, the results presented here are not
exhaustively identical to pathogen responses, which tend to be highly multi-factorial and complex, but merely resembling them through ‘mimicry’ on certain specific levels (i.e., dying cells
and neutrophils).11 Moreover, chemokine-based recruitment and TLRs/P2Rs-driven activation of neutrophil-dependent cytotoxicity is not the only distinguishing characteristic of innate immune
response to pathogens. There are several other pathogen response pathways (e.g., complement pathway, epithelial antimicrobial mechanisms)11 whose exact relationship with cell death
immunology remains enigmatic. In a physiological sense, these data have potential implications for how dying cells may modulate their immediate extracellular microenvironment. Similarly, the
intricate details of the neutrophil-dying cell interface uncovered here may have implications for various diseased scenarios, for example, cancer (owing to MTX, immunogenic apoptosis, DAMPs
and residual cells-targeted cytotoxicity), transplantation biology (concerning self/non-self model), autoimmune or inflammatory diseases (owing to nucleic acids and heightened
neutrophils' activity), and even neurodegeneration (as MTX is also a multiple sclerosis therapeutic). In future it would be crucial to uncover the overall immunological- and
disease-related implications of the cell death-associated ‘altered-self mimicry’. MATERIALS AND METHODS CELL CULTURE AND CELL DEATH INDUCTION OR TREATMENTS LLC Lewis lung carcinoma cells,
CT2A glioma cells, ID8 metastatic ovarian carcinoma cells (received from Dr. An Coosemans, KU Leuven, Belgium), B16 melanoma cells and 4T1 breast carcinoma cells were cultured at 37 °C under
5% CO2 in DMEM containing 4.5 g/l glucose and 0.11 g/l sodium pyruvate, and supplemented with 2 mM glutamine, 100 units per ml penicillin, 100 _μ_g/l streptomycin and 10% fetal calf serum
(FCS). To induce accidental necrosis, the cells were grown until the time point where other treated or untreated cells were recovered, exposed to three cycles of freezing (−80 °C) and
thawing (37 °C), and immediately utilized. Tolerogenic apoptosis was induced by TUN (TUN, 50 _μ_g/ml; Enzo Lifesciences, Farmingdale, NY, USA or Sigma-Aldrich, St. Louis, MO, USA) or
cisplatin (CDDP, 100 _μ_M; Sigma-Aldrich). Immunogenic apoptosis was induced by MTX (MTX, 2 _μ_M; Sigma, Bornem, Belgium), radiotherapy (120 Gy, performed as described previously)9, 33 or
Hypericin-based PDT (Hyp-PDT; incubation with 200 nM Hypericin, for 2 h in serum-free media, followed by light irradiation of 2.70 J/cm2 performed as described previously).17 Hypericin was
prepared, purified and stored as detailed elsewhere.17 In certain cases as applicable, the cells were treated with _E_ _scherichia_ _coli_ LPS (LPS, 1000 ng/ml; Sigma), _Salmonella
typhimurium_ flagellin (1000 ng/ml; Sigma-Aldrich), reovirus (Type 3 Dearing), parvovirus (H-1) or NDV (NDV; Hitchner B1) (the viruses were produced as described previously).19, 34 Of note,
the highest cytotoxic doses possible of reovirus, parvovirus and NDV were used for LLC cells treatments. MICE EXPERIMENTS For mice experiments, female or male C57BL/6J mice (8–10 weeks old)
were purchased from Harlan (Netherlands) or internal stock of the KU Leuven, Belgium. The animals’ care was in accordance with the institutional guidelines of University of Helsinki and/or
KU Leuven (for these and subsequent sets of mice experiments). Three million cells were killed as described above (recovered 24 h post treatment), or PBS (control/CNTR) was injected (20 _μ_l
volume) intra-dermally into the mice ear pinna. On day 1 and day 5 post injections, the mice were killed and their ear pinna was recovered. Thereafter, the pinna were minced, enzymatically
‘digested’ (1 mg/ml collagenase H (Roche, Vilvoorde, Belgium), 0.8 U/ml Dispase II (Roche) in DMEM, 2% FCS for 1 h at 37 °C), disintegrated through rigorous pippetting, sterile filtered
(through 100 and 30 _μ_m filters, BD Biosciences, Erembodegem, Belgium) and pelleted. The cells were thereafter washed, exposed to RBC lysis buffer, treated with Fc-receptor block and
stained for subsequent immunophenotyping. Following antibodies were used for immunophenotyping: anti-CD45 conjugated with APC (BD Biosciences), anti-CD11c conjugated with Alexa-488
(eBioscience, San Diego, CA, USA), anti-B220 conjugated with FITC (eBioscience), anti-Ly6G conjugated with FITC (BD Biosciences/eBioscience; RB6-8C5), anti-CD3 conjugated with Alexa-647 (BD
Biosciences), anti-F4/80 conjugated with FITC (eBioscience) and anti-CD11b conjugated with PE (eBioscience/BD Biosciences). For _in vivo_ analysis of stimulated neutrophils we used following
antibodies: Brilliant Violet 421-conjugated anti-CD11b (BD Biosciences), FITC-conjugated anti-Ly6G (BD Biosciences), PerCP Cy5.5-conjugated anti-I-A/I-E or MHC-II (BD Biosciences) and
PE-conjugated anti-CD86 (eBioscience). Blocking antibodies (and their respective isotype controls) against CXCL1/CCL2/CXCL10 (used at 75 _μ_g/ml each, 1:1:1 proportion) were purchased from
R&D Systems (Abingdon, UK). Data acquisition was performed either on BD FACSAria (Helsinki) or LSRFortessa (KU Leuven) flow cytometers (BD Biosciences) and the FlowJo software (Tree
Star, Ashland, OR, USA) was used for data analysis. Of note, a number of mice experiments was performed across two independent laboratories in two different countries (i.e., CDRT Lab, KU
Leuven, Belgium and Salven Lab, University of Helsinki, Finland). ZEBRAFISH EXPERIMENTS The zebrafish transgenic model expressing _fms_:_nfsB.mCherry_ (mCherry-labeled macrophages) and
_mpx:GFP_ (GFP-labeled neutrophils) was received from Dr. Timothy Chico, University of Sheffield, UK.22 Adult zebrafish (_Danio rerio_) were reared under standard aquaculture conditions at
28 °C on a 14/10 h light/dark cycle. Fertilized eggs were collected through natural spawning methodology. Embryos and larvae were maintained in Danieau's solution in an incubator at 28
°C. Three days post fertilization larvae were microinjected in the yolk sac with ~10–14 nl of PBS alone or PBS admixed with dead/dying cells (~50–100 cells per nl), in order to reach at
least 400–800 cells per yolk sac per larvae. The microinjections were carried out using Eppendorf FemtoJet microinjector (injection pressure of ~500 hPa). Following injections, at specific
post-injection recovery time points the larvae were sedated with a 1x tricaine solution (80 _μ_g/ml tricaine in 0.02% w/v sodium phosphate) and live larvae imaging was done using a Leica
MZ10 F stereomicroscope (Wetzlar, Germany) equipped with a DFC310 FX digital camera controlled via Leica Application Suite software (v.3.6.0, Wetzlar, Germany). The resulting images were
processed and quantified via ImageJ software (Bethesda, MD, USA). Zebrafish larvae showing no relevant fluorescence (due to possible congenital defect) or dead larvae were excluded from the
analysis. STATISTICAL ANALYSIS All statistical analyses were performed using either Prism software (GraphPad Software, San Diego, CA, USA) or GraphPad QuickCalcs online software
(http://www.graphpad.com/quickcalcs/index.cfm). The statistical analysis used is elaborated in the figure legends (significance level set at _P_<0.05). REFERENCES * Poon IK, Lucas CD,
Rossi AG, Ravichandran KS . Apoptotic cell clearance: basic biology and therapeutic potential. _Nat Rev Immunol_ 2014; 14: 166–180. Article CAS Google Scholar * Zitvogel L, Kepp O,
Kroemer G . Decoding cell death signals in inflammation and immunity. _Cell_ 2010; 140: 798–804. Article CAS Google Scholar * Garg AD, Galluzzi L, Apetoh L, Baert T, Birge RB, Bravo-San
Pedro JM _et al_. Molecular and translational classifications of DAMPs in immunogenic cell death. _Front Immunol_ 2015; 6: 588. Article Google Scholar * Medzhitov R, Janeway CA Jr .
Decoding the patterns of self and nonself by the innate immune system. _Science_ 2002; 296: 298–300. Article CAS Google Scholar * Matzinger P . The danger model: a renewed sense of self.
_Science_ 2002; 296: 301–305. Article CAS Google Scholar * Jozefowski S . The danger model: questioning an unconvincing theory. _Immunol Cell Biol_ 2016; 94: 164–168. Article Google
Scholar * Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL . Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. _Nat Med_ 2007;
13: 851–856. Article CAS Google Scholar * Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P _et al_. Consensus guidelines for the detection of immunogenic cell death.
_Oncoimmunology_ 2014; 3: e955691. Article Google Scholar * Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW _et al_. Dendritic cell vaccines based on immunogenic cell
death elicit danger signals and T cell-driven rejection of high-grade glioma. _Sci Transl Med_ 2016; 8: 328ra327. Article Google Scholar * Aaes TL, Kaczmarek A, Delvaeye T, De Craene B, De
Koker S, Heyndrickx L _et al_. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. _Cell Rep_ 2016; 15: 274–287. Article CAS Google Scholar * Janeway C
_Immunobiology: the Immune System in Health and Disease_, 6th edn. Garland Science: New York, USA, 2005.. * Kolaczkowska E, Kubes P . Neutrophil recruitment and function in health and
inflammation. _Nat Rev Immunol_ 2013; 13: 159–175. Article CAS Google Scholar * Moser B, Wolf M, Walz A, Loetscher P . Chemokines: multiple levels of leukocyte migration control. _Trends
Immunol_ 2004; 25: 75–84. Article CAS Google Scholar * Miest TS, Cattaneo R . New viruses for cancer therapy: meeting clinical needs. _Nat Rev Microbiol_ 2014; 12: 23–34. Article CAS
Google Scholar * Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T _et al_. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell
death. _EMBO J_ 2012; 31: 1062–1079. Article CAS Google Scholar * Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL _et al_. Calreticulin exposure dictates the
immunogenicity of cancer cell death. _Nat Med_ 2007; 13: 54–61. Article CAS Google Scholar * Garg AD, Elsen S, Krysko DV, Vandenabeele P, de Witte P, Agostinis P . Resistance to
anticancer vaccination effect is controlled by a cancer cell-autonomous phenotype that disrupts immunogenic phagocytic removal. _Oncotarget_ 2015; 6: 26841–26860. Article Google Scholar *
Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N _et al_. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. _J Exp Med_ 2005; 202: 1691–1701.
Article CAS Google Scholar * Koks CA, Garg AD, Ehrhardt M, Riva M, Vandenberk L, Boon L _et al_. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory
in orthotopic glioma through the induction of immunogenic cell death. _Int J Cancer_ 2015; 136: E313–E325. Article CAS Google Scholar * Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER,
Kadl A, Walk SF _et al_. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. _Nature_ 2009; 461: 282–286. Article CAS Google Scholar *
DeVries ME, Kelvin AA, Xu L, Ran L, Robinson J, Kelvin DJ . Defining the origins and evolution of the chemokine/chemokine receptor system. _J Immunol_ 2006; 176: 401–415. Article CAS
Google Scholar * Gray C, Loynes CA, Whyte MK, Crossman DC, Renshaw SA, Chico TJ . Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel
transgenic zebrafish. _Thromb Haemost_ 2011; 105: 811–819. Article CAS Google Scholar * Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB _et al_. Mycobacteria
manipulate macrophage recruitment through coordinated use of membrane lipids. _Nature_ 2014; 505: 218–222. Article CAS Google Scholar * Garg AD, Romano E, Rufo N, Agostinis P .
Immunogenic versus tolerogenic phagocytosis during anticancer therapy: mechanisms and clinical translation. _Cell Death Differ_ 2016; 23: 938–951. Article CAS Google Scholar * Sistigu A,
Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J _et al_. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. _Nat Med_ 2014; 20: 1301–1309.
Article CAS Google Scholar * de Oliveira S, Rosowski EE, Huttenlocher A . Neutrophil migration in infection and wound repair: going forward in reverse. _Nat Rev Immunol_ 2016; 16:
378–391. Article CAS Google Scholar * Thompson AJ, Locarnini SA . Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. _Immunol Cell Biol_ 2007; 85:
435–445. Article CAS Google Scholar * Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H _et al_. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune
responses through interactions between the receptor TIM-3 and the alarmin HMGB1. _Nat Immunol_ 2012; 13: 832–842. Article CAS Google Scholar * Krysko DV, Kaczmarek A, Krysko O, Heyndrickx
L, Woznicki J, Bogaert P _et al_. TLR-2 and TLR-9 are sensors of apoptosis in a mouse model of doxorubicin-induced acute inflammation. _Cell Death Differ_ 2011; 18: 1316–1325. Article CAS
Google Scholar * Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R . Tumor entrained neutrophils inhibit seeding in the premetastatic lung. _Cancer Cell_ 2011; 20: 300–314.
Article CAS Google Scholar * Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA _et al_. MET is required for the recruitment of anti-tumoural neutrophils. _Nature_
2015; 522: 349–353. Article CAS Google Scholar * Torraca V, Cui C, Boland R, Bebelman JP, van der Sar AM, Smit MJ _et al_. The CXCR3-CXCL11 signaling axis mediates macrophage recruitment
and dissemination of mycobacterial infection. _Dis Model Mech_ 2015; 8: 253–269. Article CAS Google Scholar * Vandenberk L, Garg AD, Verschuere T, Koks C, Belmans J, Beullens M _et al_.
Irradiation of necrotic cancer cells, employed for pulsing dendritic cells (DCs), potentiates DC vaccine-induced antitumor immunity against high-grade glioma. _Oncoimmunology_ 2016; 5:
e1083669. Article Google Scholar * Alkassar M, Gartner B, Roemer K, Graesser F, Rommelaere J, Kaestner L _et al_. The combined effects of oncolytic reovirus plus Newcastle disease virus
and reovirus plus parvovirus on U87 and U373 cells _in vitro_ and _in vivo_. _J Neurooncol_ 2011; 104: 715–727. Article Google Scholar Download references ACKNOWLEDGEMENTS We would like to
acknowledge Dr. Michael Dewaele and Dr. Tom Verfaillie (KU Leuven) for technical help; Dr. Shinjini Mukherjee (KU Leuven) for help with NMDS analysis. ADG is a recipient of European
Molecular Biology Organization (EMBO) Short-term Fellowship (2012–2013); and FWO Postdoctoral Fellowship (2013–2016/2016–2019) from FWO-Vlaanderen, Belgium. MVW is supported by SBO grant
(IWT-Flanders); NV is supported by FWO PhD Fellowship. This work is supported by grants from Olivia Hendrickx Research Fund to SVG, Academy of Finland and Sigrid Juselius Foundation to PS,
and FWO (G0584.12N, K202313N and GA01111N), KU Leuven (C16/15/073) and Belgian State (IAP7/32) to PA. AUTHOR CONTRIBUTIONS ADG, LV, SF, TF performed the main experiments. SVG, JM, MVW, CK,
NV provided supplementary experimental/technical support. ADG, SF, PS, PA designed the project/experiments. NG, PDW, SVG, PS provided technical/instrumental support and critically reviewed
the manuscript. ADG wrote the manuscript and made the figures. PA critically revised and corrected the manuscript. PA, PDW, PS provided senior supervision/guidance. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Department for Cellular and Molecular Medicine, Cell Death Research & Therapy (CDRT) Lab, KU Leuven University of Leuven, Leuven, Belgium Abhishek D Garg,
Sofie Van Eygen & Patrizia Agostinis * Petri Salven Lab, Haartman Institute, University of Helsinki, Helsinki, Finland Abhishek D Garg * Department of Microbiology and Immunology,
Laboratory of Pediatric Immunology, KU Leuven University of Leuven, Leuven, Belgium Lien Vandenberk & Carolien Koks * Department of Pathology, Haartman Institute, University of Helsinki,
Helsinki, Finland Shentong Fang, Tekele Fasche & Petri Salven * Department of Pharmaceutical Sciences, Laboratory for Molecular Biodiscovery, KU Leuven University of Leuven, Leuven,
Belgium Jan Maes, Niels Vanthillo & Peter de Witte * Research Group Experimental Neurosurgery and Neuroanatomy, KU Leuven University of Leuven, Leuven, Belgium Matthias Van Woensel *
Laboratoire de Pharmacie Galenique et de Biopharmacie, ULB, Brussels, Belgium Matthias Van Woensel * Department of Pediatric Oncology and Hematology, Medical School, Saarland University,
Homburg, Germany Norbert Graf * Immunologisch Onkologisches Zentrum Koln (IOZK), Cologne, Germany Stefaan Van Gool Authors * Abhishek D Garg View author publications You can also search for
this author inPubMed Google Scholar * Lien Vandenberk View author publications You can also search for this author inPubMed Google Scholar * Shentong Fang View author publications You can
also search for this author inPubMed Google Scholar * Tekele Fasche View author publications You can also search for this author inPubMed Google Scholar * Sofie Van Eygen View author
publications You can also search for this author inPubMed Google Scholar * Jan Maes View author publications You can also search for this author inPubMed Google Scholar * Matthias Van
Woensel View author publications You can also search for this author inPubMed Google Scholar * Carolien Koks View author publications You can also search for this author inPubMed Google
Scholar * Niels Vanthillo View author publications You can also search for this author inPubMed Google Scholar * Norbert Graf View author publications You can also search for this author
inPubMed Google Scholar * Peter de Witte View author publications You can also search for this author inPubMed Google Scholar * Stefaan Van Gool View author publications You can also search
for this author inPubMed Google Scholar * Petri Salven View author publications You can also search for this author inPubMed Google Scholar * Patrizia Agostinis View author publications You
can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Abhishek D Garg or Patrizia Agostinis. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no conflict of interest. ADDITIONAL INFORMATION Edited by M Piacentini Supplementary Information accompanies this paper on _Cell Death and Differentiation_ website SUPPLEMENTARY
INFORMATION SUPPLEMENTARY MATERIALS (DOC 19972 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Garg, A., Vandenberk, L., Fang, S. _et al._ Pathogen
response-like recruitment and activation of neutrophils by sterile immunogenic dying cells drives neutrophil-mediated residual cell killing. _Cell Death Differ_ 24, 832–843 (2017).
https://doi.org/10.1038/cdd.2017.15 Download citation * Received: 14 November 2016 * Revised: 20 December 2016 * Accepted: 23 January 2017 * Published: 24 February 2017 * Issue Date: May
2017 * DOI: https://doi.org/10.1038/cdd.2017.15 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative