Mcl-1 antagonizes bax/bak to promote effector cd4+ and cd8+ t-cell responses
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Members of the Bcl-2 family have critical roles in regulating tissue homeostasis by modulating apoptosis. Anti-apoptotic molecules physically interact and restrain pro-apoptotic
family members preventing the induction of cell death. However, the specificity of the functional interactions between pro- and anti-apoptotic Bcl-2 family members remains unclear. The
pro-apoptotic Bcl-2 family member Bcl-2 interacting mediator of death (Bim) has a critical role in promoting the death of activated, effector T cells following viral infections. Although
Bcl-2 is an important Bim antagonist in effector T cells, and Bcl-xL is not required for effector T-cell survival, the roles of other anti-apoptotic Bcl-2 family members remain unclear.
Here, we investigated the role of myeloid cell leukemia sequence 1 (Mcl-1) in regulating effector T-cell responses _in vivo_. We found, at the peak of the response to lymphocytic
choriomeningitis virus (LCMV) infection, that Mcl-1 expression was increased in activated CD4+ and CD8+ T cells. Retroviral overexpression of Mcl-1-protected activated T cells from death,
whereas deletion of Mcl-1 during the course of infection led to a massive loss of LCMV-specific CD4+ and CD8+ T cells. Interestingly, the co-deletion of Bim failed to prevent the loss of
Mcl-1-deficient T cells. Furthermore, lck-driven overexpression of a Bcl-xL transgene only partially rescued Mcl-1-deficient effector T cells suggesting a lack of redundancy between the
family members. In contrast, additional loss of Bax and Bak completely rescued Mcl-1-deficient effector T-cell number and function, without enhancing T-cell proliferation. These data suggest
that Mcl-1 is critical for promoting effector T-cell responses, but does so by combating pro-apoptotic molecules beyond Bim. SIMILAR CONTENT BEING VIEWED BY OTHERS C-MYC USES CUL4B TO
PRESERVE GENOME INTEGRITY AND PROMOTE ANTIVIRAL CD8+ T CELL IMMUNITY Article Open access 04 November 2023 BATF REGULATES PROGENITOR TO CYTOLYTIC EFFECTOR CD8+ T CELL TRANSITION DURING
CHRONIC VIRAL INFECTION Article 19 July 2021 HMGB2 REGULATES THE DIFFERENTIATION AND STEMNESS OF EXHAUSTED CD8+ T CELLS DURING CHRONIC VIRAL INFECTION AND CANCER Article Open access 13
September 2023 MAIN Bcl-2 family members have critical roles in immune system homeostasis. Indeed, genetic loss of the pro-apoptotic molecule Bcl-2-interacting mediator of death (Bim) or
loss of both Bax and Bak results in lymphadenopathy.1, 2 Conversely, genetic loss of the anti-apoptotic molecules Bcl-2 or myeloid cell leukemia sequence 1 (Mcl-1) results in profound
depletion of hematopoietic cells as well as developing thymocytes and peripheral T cells.3, 4, 5, 6 However, the specific interactions between pro- and anti-apoptotic Bcl-2 family members
and how these relationships control lymphocyte homeostasis remain unclear. During infection, T cells engage foreign antigen resulting in a proliferative expansion of antigen-specific
effector T cells. After antigen elimination, the majority of these effector T cells die by apoptosis, whereas some survive and become memory cells.7 This culling of activated T cells is
critical for restoring T-cell homeostasis, preventing autoimmunity, and promoting protective immune responses. Although initial work suggested a role for the death receptor pathway, more
recent work has shown a dominant role of the mitochondrial pathway of apoptosis, governed by Bcl-2 family members, in regulating the death of activated T cells.7, 8 Indeed, the BH3-only
molecule Bim, acting through Bax/Bak, is required for the apoptosis of most effector T cells.2, 9, 10, 11, 12, 13 Genetic ablation of Bim prevents the loss of effector CD4+ and CD8+ T cells
and enhances protective immunity.12, 14 However, the molecular mechanism(s) by which Bim is normally antagonized to promote effector T-cell survival remains unclear. The expression of Bcl-2
family members is dynamically regulated in activated T cells. During T-cell activation, the levels of Bcl-2 are decreased, whereas Bcl-xL expression is increased.13, 15, 16 However, despite
the normal induction of Bcl-xL, T cell-specific genetic deletion of Bcl-xL did not exacerbate the contraction of CD4+ or CD8+ T cell responses,17 suggesting that either Bcl-xL is not
required for effector T-cell survival or that it is redundant with other anti-apoptotic Bcl-2 family members. In the context of effector CD8+ T-cell subpopulations, KLRG-1hi cells have low
expression of Bcl-2, whereas KLRG-1low effector cells have high expression of Bcl-2.13 This high level of Bcl-2 expression in KLRG-1low cells is critical for their resistance to Bim-mediated
death and their survival into the memory compartment.13 Bcl-2 levels in activated T cells are controlled by IL-7 and IL-15 signaling through STAT5, a molecule essential for effector CD8+
T-cell survival.18 However, the role of other anti-apoptotic Bcl-2 family members in effector T-cell apoptosis remains unclear. Mcl-1 is another Bcl-2 family member that is highly expressed
in T cells.6, 19 Mcl-1 is critical for naive T-cell survival and Mcl-1 can efficiently bind to Bim _in vitro_ and _in vivo_.6 A recent report suggested that, at least _in vitro_, Mcl-1 may
be an important antagonist of Bim in naive T cells.20 Here, we investigated the role of Mcl-1 in maintaining activated T cells following lymphocytic choriomeningitis virus (LCMV) infection
in mice. The loss of Mcl-1 during viral infection led to a massive loss of antigen-specific CD4+ and CD8+ T cells. Notably, the loss of effector T cells in Mcl-1-deleted mice was restored by
the additional loss of Bax and Bak and was only partially restored by Bcl-xL overexpression, but was not restored by the additional loss of Bim. Together with our previous work on Bcl-2
being a major antagonist for Bim,13, 21 these data show a complex specificity between anti- and pro-apoptotic Bcl-2 family members in controlling T-cell fate. RESULTS DIVERGENT EXPRESSION OF
MCL-1 AND BCL-2 IN EFFECTOR CD4+ AND CD8+ T CELLS During T-cell activation, Bcl-2 levels are decreased in both CD4+ and CD8+ T cells, whereas Bcl-xL levels are increased.10, 19, 22, 23 A
recent report showed that Mcl-1 levels are increased following _in vitro_ T-cell activation,24 although whether or not Mcl-1 levels are changed in T cells activated _in vivo_ remains
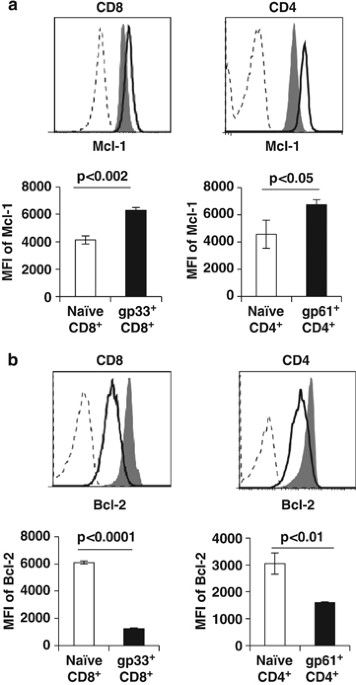
unclear. We examined expression of Mcl-1 within antigen-specific CD4+ and CD8+ T cells after infection with LCMV. At 8 days after infection, Mcl-1 levels were increased within LCMV-sp. CD4+
and CD8+ T cells, as assessed by intracellular flow cytometric analysis of MHC-tetramer+ cells (Figure 1a). In contrast, Bcl-2 levels were decreased in both LCMV-sp. CD4+ and CD8+ T cells
(Figure 1b). Together, these data suggest that Mcl-1 could be a survival factor for activated T cells, particularly when Bcl-2 levels are low. MCL-1 IS CRITICAL FOR SURVIVAL OF ACTIVATED T
CELLS _IN VIVO_ As Mcl-1 levels were increased in activated T cells, we next determined whether sustained Mcl-1 overexpression can promote effector T-cell survival. To obtain a large
population of _in vivo_ activated T cells for retroviral transduction, we injected V_β_8.2 TCR Tg (V_β_DO) mice with staphylococcal enterotoxin B (SEB) and 24 h later, transduced purified
lymph node T cells with either empty retrovirus or retroviruses overexpressing Bcl-2 or Mcl-1 and tracked the survival of transduced (Thy1.1+), SEB-reactive, V_β_8-bearing T cells. Similar
to overexpression of Bcl-2, retroviral overexpression of Mcl-1 afforded significant protection of activated T cells after 24 h culture _in vitro_ (Figure 2a). We next determined whether
Mcl-1 was required for the generation of an effector T-cell response. Conventional Mcl-1-deficient embryos suffer from maturation defects at the blastomere stage along with defects in
blastocyst peri-implantation.25 Further, T-cell-specific deletion of Mcl-1 results in massive lymphopenia due to reduced survival of thymocytes and peripheral naive T cells.6 To avoid these
issues, we used a system in which Cre expression is controlled by the αIFN-inducible Mx1-promoter, to inducibly delete Mcl-1 during LCMV infection, as we have previously done to investigate
the role of STAT5 in effector T-cell survival.18 Mx1Cre-Mcl-1f/f and Mcl-1f/f mice were infected with LCMV and their T-cell response assessed using class I and class II MHC tetramers. At day
5 after infection, although the frequency of tetramer+ CD4+ and CD8+ T cells in Mx1Cre-Mcl-1f/f mice were slightly increased, the total numbers of tetramer+ T cells in Mx1Cre-Mcl-1f/f mice
were slightly decreased when compared with control mice (Figure 2b). This difference is likely due to a loss of total CD4+ and CD8+ T cells upon Mcl-1 deletion (Supplementary Figure 1). By
day 8, both the frequency and total numbers of LCMV-sp CD4+ and CD8+ T cells in Mx1Cre-Mcl-1f/f mice was significantly reduced when compared with control mice (Figure 2b). We next examined
the deletion of Mcl-1 in Mx1Cre-Mcl-1f/f mice by intracellular flow cytometry. Although Mcl-1 levels were decreased on day 5 after infection, by day 8 Mcl-1 levels in LCMV-sp. T cells were
similar between Mx1Cre-Mcl-1f/f mice and controls (Figure 2c). We considered that the Mcl-1-induced loss of T cells may prevent control of viral infection and drive T-cell exhaustion, so we
assessed viral load by plaque assay. On day 5 after infection, viral load was slightly higher in Mx1Cre-Mcl-1f/f mice, but by day 8, the virus was undetectable in the livers of both
Mx1cre-Mcl-1f/f and Mcl-1f/f mice (Supplementary Figure 2). Thus, Mx1Cre-induced deletion of Mcl-1 led to massive loss of LCMV-sp. T cells, and selection for Mcl-1-expressing cells (that
failed to delete Mcl-1) that were able to clear the infection. CONCOMITANT LOSS OF BIM DOES NOT RESCUE MCL-1-DEFICIENT LCMV-SP. T CELLS Mcl-1 can physically associate with Bim6 and has been
shown to antagonize Bim in T cells activated _in vitro_.20 To test whether additional loss of Bim could promote survival of Mcl-1-deficient cells, we bred Mcl-1-deficient mice to Bimf/f
mice.26 First, we administered polyI:C to groups of naive control, Mx1Cre-Mcl1f/f, and Mx1Cre-Mcl-1f/fBimf/f mice. PolyI:C induced a significant loss of CD4+ and CD8+ T cells in
Mx1Cre-Mcl1f/f mice and the additional loss of Bim failed to rescue this T-cell loss (Supplementary Figure 3). We next examined whether Mcl-1-antagonized Bim in effector T cells by infecting
the same groups of mice with LCMV. On day 8 after LCMV infection, the frequency and total numbers of CD4+ gp61-sp. and CD8+ gp33-sp.T cells were again decreased in Mx1Cre-Mcl-1f/f mice and
the additional loss of Bim was unable to restore LCMV-sp T-cell responses (Figures 3a and b). In Mx1Cre-Bimf/f mice, the numbers and frequency of LCMV-sp. CD4+ and CD8+ T cells were similar
to controls, consistent with our and others previous data showing that the total deletion of Bim does not increase LCMV-sp. T cells at the peak of the response.11, 12 We again failed to
detect a significant loss of Mcl-1 staining in LCMV-sp. CD4+ or CD8+ T cells in Mx1Cre-Mcl-1f/f mice (Figure 3c). To assess Bim deletion, we measured Bim expression within tetramer+ T cells
by intracellular flow cytometry using a Bim-specific antibody.27 Notably, we found substantial loss of Bim in CD4+gp61-sp and CD8+ gp33-sp T cells from Mx1Cre-Mcl-1f/f Bimf/f mice (Figure
3c). Thus, despite efficient deletion, this additional loss of Bim failed to restore either naive or LCMV-sp. CD4+ and CD8+ T-cell responses in mice that had inducibly deleted Mcl-1,
suggesting that Mcl-1 antagonizes additional pro-apoptotic molecules in T cells. BCL-XL OVEREXPRESSION PARTIALLY RESTORES CD4+, BUT NOT CD8+ T CELLS IN INDUCIBLE MCL-1-DELETED MICE To assess
whether overexpression of Bcl-xL could substitute for Mcl-1 deletion and rescue LCMV-sp. T-cell responses, we bred lck-Bcl-xL Tg mice to Mx1Cre-Mcl-1f/f mice, infected them with LCMV, and
assessed their T-cell responses on day 8 after infection. Bcl-xL levels, as assessed by intracellular flow cytometry were increased in both LCMV-sp. CD4+ and CD8+ T cells, although the
increase was greater in CD4+gp61-sp. T cells (Figure 4a). Surprisingly, the frequency and total numbers of CD8+ gp33-sp. T cells in Mx1Cre-Mcl-1f/f mice were not restored by expression of
ectopic Bcl-xL (Figures 4b and c). However, the frequency and total numbers of CD4+ gp61-sp. T cells were partially restored by Bcl-xL overexpression, but not back to control levels (Figures
4b and c). We observed a subtle (<20%) decrease in Mcl-1 levels in LCMV-sp. CD4+ and CD8+ T cells from lck-Bcl-xLMx1Cre-Mcl-1f/f mice (data not shown). Thus, even the overexpression of
Bcl-xL is unable to compensate for the Mcl-1 deletion in activated CD8+ T cells and only partially compensates in CD4+ T cells _in vivo._ LEVELS OF PUMA, BIM, AND NOXA ARE INCREASED IN
LCMV-SP. EFFECTOR T CELLS FROM MX1CRE-MCL1F/F MICE As concomitant loss of Bim was unable to restore LCMV-sp. T cells in Mx1Cre-Mcl-1f/f mice, we considered the possibility that Mcl-1 may
antagonize multiple BH3-only molecules. We found that the levels of Bim, Puma, and Noxa were all expressed in naive CD4+ and CD8+ T cells (Figure 5). Specificity of the Puma antibody was
confirmed on Puma-deficient mice (Supplementary Figure 4). Following LCMV infection, the levels of Bim and Puma were slightly decreased within effector CD4+ and CD8+ T cells (Figure 5),
whereas Noxa was increased in LCMV-sp. CD4+, but not CD8+, T cells (Figure 5). Nonetheless, activated T cells express several BH3-only molecules, and the loss of Bim alone is not sufficient
to restore T cells in Mcl-1-deficient mice. CONCOMITANT LOSS OF BAX AND BAK RESTORES MCL-1-DEFICIENT EFFECTOR T CELLS Neither the single additional loss of Bim nor the overexpression of
Bcl-xL, substantially restored effector T-cell responses in Mcl-1-deleted mice, suggesting that Mcl-1 may target multiple BH3-only molecules in a manner independent of Bcl-xL or that Mcl-1
targets the downstream apoptotic effectors Bax and Bak. Alternatively, we recently showed that an isoform of Mcl-1 contributes to mitochondrial respiration and ATP generation,28 raising the
possibility that Mcl-1 may function in a non-apoptotic manner to control T-cell responses. To distinguish between these possibilities, we generated mice lacking Bak that were also
conditionally deficient in Bax and Mcl-1. The additional loss of Bax and Bak would prevent the induction of apoptosis upon loss of Mcl-1, but would not rescue mitochondrial function.
Mx1Cre-Mcl-1f/f and Mx1Cre-Mcl-1f/fBaxf/fBak−/− mice (along with the respective controls) were infected with LCMV, killed at day 8 of infection and the numbers of LCMV-sp. T cells were
assessed with MHC tetramers. Inducible deletion of Mcl-1 resulted again in decreased CD4+ gp61-sp. and CD8+ gp33-sp. T-cell responses; however, in Mx1Cre-Mcl-1f/fBaxf/fBak−/− mice, the
frequency and total numbers of LCMV-sp. CD4+ and CD8+ T cells were restored (Figures 6a and b). Further, the loss of both Bax and Bak allowed the survival and persistence effector T cells
with decreased Mcl-1 expression indicating that the deletion of both pro-apoptotic effectors relieves the need of Mcl-1 (Figure 6c). To determine whether the restoration of Mcl-1-deleted
effector T cells by the additional loss of Bax and Bak was due to increased proliferation of these cells, we injected the mice with BrdU and assessed BrdU incorporation in tetramer+ T cells.
The frequency of LCMV-sp. CD4+ and CD8+ T cells that were BrdU+ was higher in Mx1Cre-Mcl-1f/fBaxf/fBak−/− mice compared with Mx1Cre-Baxf/fBak−/− mice (Figure 6d). However, the 2–4%
difference in the percentage of BrdU+ T cells is insufficient to account for the three- to sixfold increase in T cells rescued by the combined absence of Bax and Bak. Importantly, the
effector T cells retained functionality, as similar frequencies of IFN-_γ_/TNF-α-producing T cells were observed in mice that had or had not inducibly deleted all three genes (Supplementary
Figure 5). Further, virus was undetectable in the livers of mice with deletions of Mcl-1, Bax, and Bak on day 8 after infection (data not shown). Thus, the absence of both Bax and Bak
promoted the survival and functionality of effector CD4+ and CD8+ effector T cells following inducible Mcl-1 deletion. MIXED BONE MARROW (BM) CHIMERAS REVEAL A CELL INTRINSIC EFFECT OF MCL-1
ON T-CELL SURVIVAL One caveat of the Mx1Cre-system is that during the course of the response, Mcl-1 is being deleted in multiple type I interferon responsive tissues and not just in T
cells.5 To circumvent this issue, we generated mixed BM chimeras using BM from CD45.1+ B6.SJL-_Ptprc__a_ _Pepc__b_/BoyJ (referred to as CD45.1 congenics) and CD45.2+ Mx1Cre-Mcl-1f/f mice or
from CD45.1 congenics and Mcl-1f/f as controls at a 50 : 50 ratio into lethally irradiated CD45.1 congenic recipients (Figure 7a). Before infection, there was a reduced engraftment of
Mx1Cre-Mcl1f/f cells in the peripheral blood of mixed BM chimeras (Figure 7b). Nine weeks after reconstitution, mice were infected with LCMV and the numbers of control CD45.1 cells _versus_
Mx1Cre-Mcl-1f/f CD45.2, LCMV-sp. CD4+ and CD8+ T cells were enumerated on day 8 after infection. In control mice, the numbers of CD8+ gp33-sp. and CD4+ GP61-sp. T cells derived from CD45.2
Mcl-1f/f mice were slightly decreased compared with those derived from CD45.1 congenics (Figure 7c), likely because of the slightly lower CD45.2 chimerism observed in these animals (Figure
7b). In contrast, the numbers of CD8+ gp33-sp. and CD4+ gp61-sp. derived from CD45.2 Mx1Cre-Mcl-1f/f BM were decreased when compared the same cells derived from CD45.1 congenics (Figures 7c
and d). Although the chimerism was lower in this group (Figure 7b), there was a significant loss of both CD8+ gp33-sp. and CD4+ gp61-sp. derived from CD45.2 Mx1Cre-Mcl-1f/f BM compared with
their CD45.1 congenic controls (Figures 7c and d). The few tetramer+ cells emerging from the CD45.2 Mx1Cre-Mcl-1f/f BM exhibited a slight decrease in Mcl-1 levels compared with controls
(Figure 7e). Together, these data demonstrate that Mcl-1 is required in a cell intrinsic manner for generation of LCMV-sp. CD4+ and CD8+ T-cell responses. DISCUSSION T cells express multiple
pro- and anti-apoptotic Bcl-2 family members, however, the interactions between individual Bcl-2 family members and their specific roles in maintaining T-cell homeostasis has remained
unclear. Initial work, using BH3 peptides from BH3-only pro-apoptotic Bcl-2 family members indicated that Bim and Puma could bind to nearly all anti-apoptotic molecules, whereas Noxa and Bad
were more selective, Bad bound to Bcl-2, Bcl-xL, and Bcl-w but not A1 or Mcl-1 and Noxa had a higher affinity for Mcl-1 and A1 but not for Bcl-2, Bcl-xL, or Bcl-w.29 These data are
consistent with the function of ABT-737, a BH3-mimetic based on the BH3 domain of Bad, which targets Bcl-2, Bcl-xL, and Bcl-w, but not A1 or Mcl-1.30 We previously showed that that Mcl-1 is
a critical survival molecule for promoting naive T-cell survival _in vivo_6 and others have found that Mcl-1 is critical for activated and memory T-cell survival _in vitro_.24 Mcl-1 also
possesses another function, to ensure appropriate mitochondrial respiration,28 and it was possible that this function of Mcl-1 contributed to T-cell homeostasis. However, our ability to
rescue T-cell responses by the additional loss of Bax and Bak demonstrates that the anti-apoptotic function of Mcl-1 contributes significantly to its ability to maintain T-cell homeostasis.
Both biochemical and genetic experiments have suggested an interaction between Bim and Mcl-1 in naive T cells6, 20 and other cells,31, 32 although the _in vivo_ functionality of this
interaction has not been assessed. Our data show that the _in vivo_ deletion of Bim fails to rescue Mcl-1-deficient cells, whereas the loss of Bax and Bak is sufficient to rescue CD4+ and
CD8+ T-cell responses in Mcl-1-deleted mice. We envision three possible models by which Mcl-1 protects activated T cells from death. First, Mcl-1 may act downstream of Bim, targeting the
pro-apoptotic molecules Bax and/or Bak. In support of this model, it has been shown that Mcl-1 can antagonize Bak on the mitochondria.33, 34 Furthermore, the additional loss of Bak restored
most cells when Mcl-1 was deleted _in vitro_, but only when IL-7 is present.20 However, we have failed to observe rescue of Mcl-1-deleted hematopoietic cells by the loss of Bak alone (data
not shown). Nonetheless, it is possible that loss of Mcl-1 can directly lead to the spontaneous activation of both Bax and Bak in T cells. Second, it is possible that Mcl-1 inhibits a
pro-apoptotic BH3-only member that functions independently of Bim. Indeed, our data show that activated T cells express Puma and Noxa in addition to Bim; therefore, it is possible that with
the loss of Mcl-1, Puma, Noxa, and Bim can all facilitate the activation of Bax and Bak.29, 35 The third possibility is that Mcl-1 acts as to ‘tune’ Bim-mediated death. In this scenario, Bim
is largely inhibited by another anti-apoptotic Bcl-2 family member, such as Bcl-2, and Mcl-1 buffers excess Bim that is not antagonized by Bcl-2. Thus, in activated T cells, increased Mcl-1
expression may enhance resistance to death at a time when it is critical to balance pathogen clearance with immunopathology. Consistent with this concept, it has been shown that GSK-3_β_
inhibitors can maintain Mcl-1 levels and prolong activated T-cell survival.36 The loss of Bim failed to restore Mcl-1-deficient cells, so what normally restrains Bim in T cells? We recently
showed that the loss of naive, effector, and memory CD8+ T cells in Bcl-2-deficient or ABT-737-treated mice are largely rescued by additional Bim deficiency.13, 21 A critical component to
the sparing of effector CD8+ T cells is the action of the cytokines IL-7 and IL-15 that act to drive STAT5-dependent expression of Bcl-2.18 IL-4, IL-7, and IL-15 can induce an increase in
Mcl-1 protein levels in activated T cells20 and Mcl-1 levels are largely controlled post-translationally,37 one possibility is that cytokines regulate Mcl-1’s stability. However, when we
cultured T cells with cycloheximide, neither IL-7 nor IL-15 significantly stabilized Mcl-1 levels within effector CD8+ T cells (data not shown). Further, IL-7 can increase the survival of
Mcl-1-deleted T cells to a similar extent as Bcl-2 overexpression and IL-7 failed to further improve survival of Bcl-2 Tg/Mcl-1-deficient effector T cells.20 Together, these data suggest
that a major survival function of cytokines is to promote the transcriptional upregulation of Bcl-2 rather than to modulate the turnover of Mcl-1. In summary, these data suggest that
critical and specific interactions between Bcl-2 family members control T-cell homeostasis. This is important because of the potential development of Bcl-2 antagonists being developed as
therapeutics. As these drugs are developed for combating tumors, understanding the specificity of their interactions is crucial. One such drug, ABT-737 specifically antagonizes Bcl-2,
Bcl-xL, and Bcl-w, but not A1 or Mcl-1 and is effective at tumors that express high levels of Bcl-2 or Bcl-xL, but it is ineffective against tumors expressing high levels of Mcl-1.30, 38, 39
In tumors that do not express high levels of Bcl-2, Bcl-xL, or Bcl-w, other Bcl-2 family member antagonists may be more beneficial. However, a possible complication to these inhibitors is
that they may cause lymphopenia.21 Therefore, these inhibitors may also be exploited to target specific populations of T cells (activated effector cells) when these cells are wreaking havoc
(e.g., autoimmunity). Indeed, recent studies have exploited their use in mouse models of autoimmunity with substantial benefit.40, 41 For such therapeutics to have maximal efficiency with
minimal off-target effects, gaining knowledge of the specific interactions between Bcl-2 family members in specific populations of cells is essential. Despite a large amount of literature
showing biochemical interactions between Bim and Mcl-1, we clearly show that, _in vivo_, the additional loss of Bim fails to rescue the loss of cells imposed by Mcl-1 deficiency. These data
highlight the need to more carefully define the specific and antagonistic interactions between Bcl-2 family members that are operative _in vivo_. MATERIALS AND METHODS MICE Conditional
Mcl-1f/f mice6 were crossed to Mx1Cre mice (Jackson Laboratory, Bar Harbor, ME, USA); to Lck-human Bcl-xL Tg mice (Jackson Laboratory); to Baxf/fBak−/− mice;26 and to Bimf/f mice26 (a kind
gift from the late S. Korsmeyer). Puma-deficient mice were purchased from Jackson Laboratory. V_β_DO mice express the TCR_β_ chain from the DO11.10 TCR in a B10.D2 background.10, 42
B6.SJL-_Ptprc__a_ _Pepc__b_/BoyJ (BoyJ) were purchased from Jackson Laboratory. All animal protocols were reviewed and approved by our institutional animal care and use committees. VIRUS The
Armstrong-3 strain of LCMV, described previously,43 was grown in BHK-21 cells; the number of plaque-forming units (p.f.u.) was assayed on Vero cells as described.43 Mice were injected
intraperitoneally (i.p.) with 0.25 ml of LCMV (2 × 105 p.f.u.) diluted in balanced salt solution. Determination of liver viral load was determined by plaque assay on Vero cells as
described.43 MOLECULAR BIOLOGY Mouse Mcl-1 cDNA was purchased from Addgene (Cambridge, MA, USA) and subcloned into the retroviral plasmid MiT.44 MiT-Bcl-2 was generated as described.10
Retroviruses were generated by cotransfection of HEK293 with pCLEco and the MiT plasmid of interest by using calcium phosphate as described.10 After transduction, cells were stained with
various fluorescently labeled antibodies and live and dead cells were distinguished by their forward side scatter properties using a flow cytometer as described.10 MHC TETRAMERS Class II MHC
tetrameric staining reagents were created as described.45, 46 The methodology for preparation of MHC class I tetramers was modified from the protocol described by Altman and co-workers47
and were created as described previously.12, 48 For some experiments, I-Abgp66-77 tetramers were obtained from the NIH Tetramer Core Facility. We have observed no significant difference in
tracking the LCMV-sp. CD4+ T-cell response using homemade _versus_ NIH-generated LCMV-sp. class II tetramers. FLOW CYTOMETRY Spleens were harvested and 106 cells were stained with
fluorochrome-labeled antibodies (anti-CD4, anti-CD8, anti-CD44, anti-IL-7R and anti-KLRG-1) or intracellularly with antibodies against Bcl-xL (BD Transduction Labs, San Jose, CA, USA); Mcl-1
(Rockland Immunochemical, Gilbertsville, PA, USA); Bcl-2 (made in house from hybridoma 3F11); and Bim (Cell Signaling, Danvers, MA, USA), and data acquired using an LSRII flow cytometer (BD
Biosciences, San Jose, CA, USA). Data were analyzed with FacsDIVA software (BD Biosciences). Antigen-specific CD4+ T cells were identified by staining 2 × 106 spleen cells with I-Abgp61-80
tetrameric staining reagents for 2 h at 37 °C, gating away from CD16/32+ cells as described.12 LCMV-sp. CD8+ T cells were detected by staining 2 × 106 cells/well with either Dbgp33
tetrameric staining reagents for 90 min at 4 °C followed by cell surface marker staining as described.12 Intracellular cytokine staining was performed as described.12 Briefly, spleen cells
from mice were cultured at 37 °C for 4–5 h with or without various LCMV peptides (GP61-80 10 _μ_g/ml; GP33-41, at 1 _μ_g/ml) and BrefeldinA at 10 _μ_g/ml. After culture, cells were stained
for cell surface markers (CD4, CD8, CD44, antibodies from BD Pharmingen, San Jose, CA, USA) for 45 min at 4 °C. Cells were then washed, fixed, and permeabilized with 0.03% saponin and
stained intracellularly with PE-labeled anti-IFN-_γ_ or anti-IL-2 antibody (BD Pharmingen). A minimum of 5 × 105 events were acquired on a FacsCalibur flow cytometer and analyzed using
CellQuest software (BD Biosciences). MIXED BONE MARROW CHIMERAS BM from Mcl-1f/f Mx1Cre mice (CD45.2+) was mixed 1 : 1 with BM from B6.SJL-_Ptprc__a_ _Pepc__b_/BoyJ (CD45.1+) and 5 × 106
cells injected intravenously into lethally irradiated (1100 Rads) BoyJ recipients and allowed to engraft for 8 weeks at which time peripheral blood analyses indicated stable engraftment of
both CD45.1 and CD45.2 cell surface-positive cells at a 3 : 1 ratio. The chimeric mice were then infected as described. STATISTICAL ANALYSES Statistical analyses were performed using a
Student’s two-sample _t_-test with Minitab for Windows Software (Release 14), State College, PA, USA. ABBREVIATIONS * Bim: Bcl-2-interacting mediator of death * BM: bone marrow * LCMV:
lymphocytic choriomeningitis virus * Mcl-1: myeloid cell leukemia sequence 1 * p.f.u.: plaque forming units * SEB: staphylococcal enterotoxin B REFERENCES * Bouillet P, Metcalf D, Huang DC,
Tarlinton DM, Kay TW, Kontgen F _et al_. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. _Science_ 1999; 286:
1735–1738. Article CAS PubMed Google Scholar * Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB . Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis.
_Nat Immunol_ 2002; 3: 932–939. Article CAS PubMed Google Scholar * Nakayama K, Nakayama K, Negishi I, Kuida K, Shinkai Y, Louie MC _et al_. Disappearance of the lymphoid system in Bcl-2
homozygous mutant chimeric mice. _Science_ 1993; 261: 1584–1588. Article CAS PubMed Google Scholar * Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ . Bcl-2-deficient mice demonstrate
fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. _Cell_ 1993; 75: 229–240. Article CAS PubMed Google Scholar * Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S,
Akashi K _et al_. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. _Science_ 2005; 307: 1101–1104. Article CAS PubMed Google Scholar * Opferman JT,
Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ . Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. _Nature_ 2003; 426: 671–676. Article CAS PubMed
Google Scholar * Kurtulus S, Tripathi P, Opferman JT, Hildeman DA . Contracting the 'mus cells'--does down-sizing suit us for diving into the memory pool? _Immunol Rev_ 2010; 236:
54–67. Article CAS PubMed PubMed Central Google Scholar * D’Cruz LM, Rubinstein MP, Goldrath AW . Surviving the crash: transitioning from effector to memory CD8+ T cell. _Semin
Immunol_ 2009; 21: 92–98. Article PubMed PubMed Central Google Scholar * Grayson JM, Weant AE, Holbrook BC, Hildeman D . Role of Bim in Regulating CD8+ T-cell responses during chronic
viral infection. _J Virol_ 2006; 80: 8627–8638. Article CAS PubMed PubMed Central Google Scholar * Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J _et al_. Activated
T cell death _in vivo_ mediated by proapoptotic bcl-2 family member bim. _Immunity_ 2002; 16: 759–767. Article CAS PubMed Google Scholar * Prlic M, Bevan MJ . Exploring regulatory
mechanisms of CD8+ T cell contraction. _Proc Natl Acad Sci USA_ 2008; 105: 16689–16694. Article CAS PubMed PubMed Central Google Scholar * Wojciechowski S, Jordan MB, Zhu Y, White J,
Zajac AJ, Hildeman DA . Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. _Eur J Immunol_ 2006; 36: 1694–1706. Article CAS PubMed PubMed Central Google
Scholar * Kurtulus S, Tripathi P, Moreno-Fernandez ME, Sholl A, Katz JD, Grimes HL _et al_. Bcl-2 allows effector and memory CD8+ T cells to tolerate higher expression of Bim. _J Immunol_
2011; 186: 5729–5737. Article CAS PubMed Google Scholar * Reckling S, Divanovic S, Karp CL, Wojciechowski S, Belkaid Y, Hildeman D . Proapoptotic Bcl-2 family member Bim promotes
persistent infection and limits protective immunity. _Infect Immun_ 2008; 76: 1179–1185. Article CAS PubMed Google Scholar * Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R .
Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. _Nat Immunol_ 2003; 4: 1191–1198. Article CAS PubMed Google
Scholar * Mitchell T, Kappler J, Marrack P . Bystander virus infection prolongs activated T cell survival. _J Immunol_ 1999; 162: 4527–4535. CAS PubMed Google Scholar * Zhang N, He YW .
The antiapoptotic protein Bcl-xL is dispensable for the development of effector and memory T lymphocytes. _J Immunol_ 2005; 174: 6967–6973. Article CAS PubMed Google Scholar * Tripathi
P, Kurtulus S, Wojciechowski S, Sholl A, Hoebe K, Morris SC _et al_. STAT5 is critical to maintain effector CD8+ T cell responses. _J Immunol_ 2010; 185: 2116–2124. Article CAS PubMed
Google Scholar * Hildeman D, Jorgensen T, Kappler J, Marrack P . Apoptosis and the homeostatic control of immune responses. _Curr Opin Immunol_ 2007; 19: 516–521. Article CAS PubMed
PubMed Central Google Scholar * Dunkle A, Dzhagalov I, He YW . Cytokine-dependent and cytokine-independent roles for Mcl-1: genetic evidence for multiple mechanisms by which Mcl-1 promotes
survival in primary T lymphocytes. _Cell Death Dis_ 2011; 2: e214. Article CAS PubMed PubMed Central Google Scholar * Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz
JD _et al_. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. _J Exp Med_ 2007; 204: 1665–1675. Article CAS PubMed PubMed Central Google Scholar * Boise
LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T _et al_. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. _Immunity_ 1995; 3: 87–98. Article
CAS PubMed Google Scholar * Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, Marrack P . Control of Bcl-2 expression by reactive oxygen species. _Proc Natl Acad Sci USA_
2003; 100: 15035–15040. Article CAS PubMed PubMed Central Google Scholar * Dzhagalov I, Dunkle A, He YW . The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at
multiple stages. _J Immunol_ 2008; 181: 521–528. Article CAS PubMed Google Scholar * Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ . Mcl-1 deficiency results in
peri-implantation embryonic lethality. _Genes Dev_ 2000; 14: 23–27. CAS PubMed PubMed Central Google Scholar * Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ . Essential
role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. _Proc Natl Acad Sci USA_ 2005; 102: 11272–11277. Article CAS PubMed PubMed Central Google Scholar * Chougnet
CA, Tripathi P, Lages CS, Raynor J, Sholl A, Fink P _et al_. A major role for Bim in regulatory T cell homeostasis. _J Immunol_ 2011; 186: 156–163. Article CAS PubMed Google Scholar *
Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M _et al_. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. _Nat
Cell Biol_ 2012; 14: 575–583. Article CAS PubMed PubMed Central Google Scholar * Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG _et al_. Differential targeting of prosurvival
Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. _Mol Cell_ 2005; 17: 393–403. Article CAS PubMed Google Scholar * Oltersdorf T, Elmore SW, Shoemaker
AR, Armstrong RC, Augeri DJ, Belli BA _et al_. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. _Nature_ 2005; 435: 677–681. Article CAS PubMed Google Scholar *
Han J, Goldstein LA, Gastman BR, Froelich CJ, Yin XM, Rabinowich H . Degradation of Mcl-1 by granzyme B: implications for Bim-mediated mitochondrial apoptotic events. _J Biol Chem_ 2004;
279: 22020–22029. Article CAS PubMed Google Scholar * Jamil S, Wang SW, Bondy L, Mojtabavi S, Duronio V . Prevention of cytokine withdrawal-induced apoptosis by Mcl-1 requires
interaction between Mcl-1 and Bim. _Biochem Cell Biol_ 2010; 88: 809–818. Article CAS PubMed Google Scholar * Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M _et al_. tBID, a
membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. _Genes Dev_ 2000; 14: 2060–2071. CAS PubMed PubMed Central Google Scholar * Willis SN, Chen L, Dewson G, Wei A,
Naik E, Fletcher JI _et al_. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. _Genes Dev_ 2005; 19: 1294–1305. Article CAS PubMed
PubMed Central Google Scholar * Zhong Q, Gao W, Du F, Wang X . Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. _Cell_ 2005;
121: 1085–1095. Article CAS PubMed Google Scholar * Sengupta S, Jayaraman P, Chilton PM, Casella CR, Mitchell TC . Unrestrained glycogen synthase kinase-3 beta activity leads to
activated T cell death and can be inhibited by natural adjuvant. _J Immunol_ 2007; 178: 6083–6091. Article CAS PubMed Google Scholar * Stewart DP, Koss B, Bathina M, Perciavalle RM,
Bisanz K, Opferman JT . Ubiquitin-independent degradation of antiapoptotic MCL-1. _Mol Cell Biol_ 2010; 30: 3099–3110. Article CAS PubMed PubMed Central Google Scholar * Konopleva M,
Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S _et al_. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. _Cancer Cell_ 2006; 10:
375–388. Article CAS PubMed Google Scholar * van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE _et al_. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and
efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. _Cancer Cell_ 2006; 10: 389–399. Article CAS PubMed PubMed Central Google Scholar * Bardwell PD, Gu J, McCarthy D,
Wallace C, Bryant S, Goess C _et al_. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. _J Immunol_ 2009; 182: 7482–7489. Article CAS
PubMed Google Scholar * Carrington EM, Vikstrom IB, Light A, Sutherland RM, Londrigan SL, Mason KD _et al_. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent
another class of selective immune modulatory drugs. _Proc Natl Acad Sci USA_ 2010; 107: 10967–10971. Article CAS PubMed PubMed Central Google Scholar * Fenton RG, Marrack P, Kappler JW,
Kanagawa O, Seidman JG . Isotypic exclusion of gamma delta T cell receptors in transgenic mice bearing a rearranged beta-chain gene. _Science_ 1988; 241: 1089–1092. Article CAS PubMed
Google Scholar * Hildeman D, Yanez D, Pederson K, Havighurst T, Muller D . Vaccination against persistent viral infection exacerbates CD4+ T-cell-mediated immunopathological disease. _J
Virol_ 1997; 71: 9672–9678. CAS PubMed PubMed Central Google Scholar * Mitchell TC, Hildeman D, Kedl RM, Teague TK, Schaefer BC, White J _et al_. Immunological adjuvants promote
activated T cell survival via induction of Bcl-3. _Nat Immunol_ 2001; 2: 397–402. Article CAS PubMed Google Scholar * Crawford F, Kozono H, White J, Marrack P, Kappler J . Detection of
antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. _Immunity_ 1998; 8: 675–682. Article CAS PubMed Google Scholar * Rees W, Bender J, Teague TK,
Kedl RM, Crawford F, Marrack P _et al_. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses _in vivo_ and _in vitro_. _Proc Natl Acad Sci
USA_ 1999; 96: 9781–9786. Article CAS PubMed PubMed Central Google Scholar * Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI _et al_. Phenotypic analysis of
antigen-specific T lymphocytes. _Science_ 1996; 274: 94–96. Article CAS PubMed Google Scholar * Fuller MJ, Zajac AJ . Ablation of CD8 and CD4 T cell responses by high viral loads. _J
Immunol_ 2003; 170: 477–486. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank the Hildeman and Opferman labs for helpful suggestions and comments. This
work was supported by Public Health Service Grants AI057753 and DK081175 (to DAH) and HL102175, the American Cancer Society 119130-RSG-10-255-01-LIB, a Cancer Center Support Grant
P30CA021765, and the American Lebanese Syrian Associated Charities of St. Judes Children’s Research Hospital (to JTO). AUTHOR CONTRIBUTIONS JTO and DAH designed the research; PT and BK
performed the research; DAH, JTO, BK, and PT analyzed and interpreted the data; PT performed the statistical analysis; and JTO and DAH wrote the manuscript. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Division of Cellular and Molecular Immunology, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine,
Cincinnati, Ohio, USA P Tripathi & D A Hildeman * Department of Biochemistry, St. Jude’s Children’s Research Hospital, Memphis, Tennessee, USA B Koss & J T Opferman Authors * P
Tripathi View author publications You can also search for this author inPubMed Google Scholar * B Koss View author publications You can also search for this author inPubMed Google Scholar *
J T Opferman View author publications You can also search for this author inPubMed Google Scholar * D A Hildeman View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHORS Correspondence to J T Opferman or D A Hildeman. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION
Edited by C Borner Supplementary Information accompanies this paper on Cell Death and Differentiation website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES (PDF 306 KB) RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tripathi, P., Koss, B., Opferman, J. _et al._ Mcl-1 antagonizes Bax/Bak to promote effector CD4+ and CD8+ T-cell
responses. _Cell Death Differ_ 20, 998–1007 (2013). https://doi.org/10.1038/cdd.2013.25 Download citation * Received: 23 October 2012 * Revised: 26 February 2013 * Accepted: 28 February 2013
* Published: 05 April 2013 * Issue Date: August 2013 * DOI: https://doi.org/10.1038/cdd.2013.25 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
KEYWORDS * T cells * Mcl-1 * Bim * Bax * Bak * Bcl-2