Is there an expiration date for a cord blood unit in storage?
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Access through your institution Buy or subscribe With improving expertise and supportive care, cord blood (CB) transplants are now associated with outcomes comparable to unrelated and
sibling donor transplants.1 There is a need to increase the size and diversity of international CB inventories and to retain cryopreserved CB units for as long as safely possible. There are
_in vitro_ data supporting >90% recovery of hematopoietic progenitor cells from frozen CB cells stored for up to 12 years,2 and data showing that progenitor cell recoveries from
short-term freeze samples ranging from 2 to 8 weeks are comparable to those cryopreserved for 10–15 years.3,4 Broxmeyer _et al._5 reported adequate recovery (80–100%) of
granulocyte–macrophage and multi-potential hematopoietic progenitors from functional CB units cryopreserved for up to 23.5 years as well as recovery of viable progenitor cells from CBs
frozen for 15 years, which were able to generate CD45+ human cell engraftment when infused into sublethally irradiated NOD-SCID mice and were comparable to that reported with fresh CB.6
Despite this _in vitro_ evidence, transplant physicians still remain concerned about the possible loss of integrity of frozen CB units associated with longer durations of storage.7 In one
report, the incidence of bag breaks over a 6.5-year period was 3.5%, where 75% of the breaches occurred in units that had been cryopreserved for >2 years.7 Therefore, it is important to
determine whether prolonged time of cryopreservation and storage may adversely affect clinical transplant outcomes, and with the new US Food and Drug Administration licensing regulations for
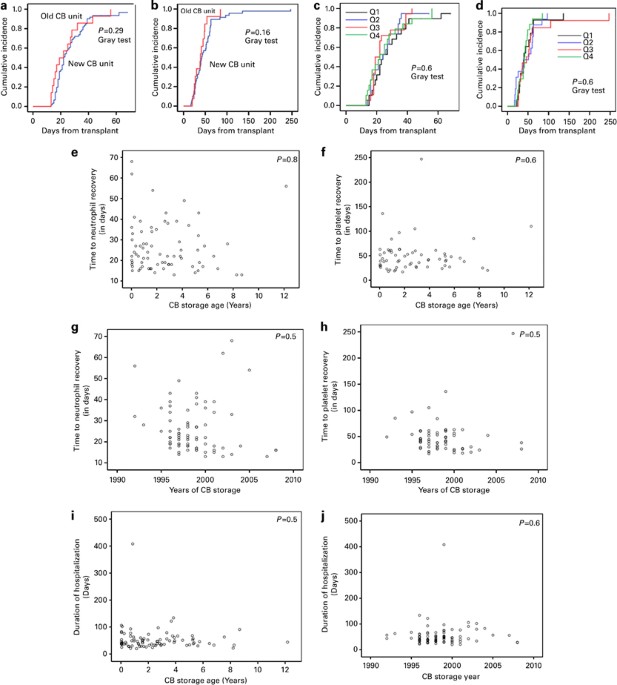
CB units this question has become even more relevant: 'is there an expiration date for CB unit in storage?'. We reviewed 86 consecutive single CB transplant recipients in the
period from March 1996 to June 2011, where 15 patients received CB units older than 5 years (CB age 12.2 years=1; >8 years=3; >7 years= 1; >6 years=2; >5 years=8). The vast
majority of the CB units were obtained from NMDP (National Marrow Donor Program) or Netcord Banks (American Red Cross Portland=1; AUNCB=1; Australia=1; Barcelona=5; Belgium=5; Bergan
Paramus, NJ, USA=1; Caitlin Raymond, UK=1; NC, USA=1; Colorado=9; Dusseldorf=10; France=1; JP McCarthy, USA=1; London=5; MD Anderson=2; Italy=9; NYCB=16; Puget Sound, OR, USA=1; Spain=2; St
Louis=13; Tokyo=1). All CB units underwent washing and RBC depletion before freezing. The median length of storage of CB units was 2 years (range, 0.03–12 years). For the purpose of
analysis, duration of CB unit storage was divided into four equal quartiles as well as an arbitrary cutoff of 5 years based on the common practice adopted by some public and private CB
banks. Standard thawing procedures were followed at the MD Anderson Cell Therapy Laboratory.8 The pre-infusion viabilities were >90%. Patient and CB unit characteristics are described in
Table 1. This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online
access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which
are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support REFERENCES * Laughlin MJ, Eapen M,
Rubinstein P, Wagner JE, Zhang MJ, Champlin RE _et al_. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. _N Engl J Med_ 2004; 351:
2265–2275. Article CAS Google Scholar * Mugishima H, Harada K, Chin M, Suzuki T, Takagi K, Hayakawa S _et al_. Effects of long-term cryopreservation on hematopoietic progenitor cells in
umbilical cord blood. _Bone Marrow Transplant_ 1999; 23: 395–396. Article CAS Google Scholar * Kobylka P, Ivanyi P, Breur-Vriesendorp BS . Preservation of immunological and colony-forming
capacities of long-term (15 years) cryopreserved cord blood cells. _Transplantation_ 1998; 65: 1275–1278. Article CAS Google Scholar * Yamamoto S, Ikeda H, Toyama D, Hayashi M, Akiyama
K, Suzuki M _et al_. Quality of long-term cryopreserved umbilical cord blood units for hematopoietic cell transplantation. _Int J Hematol_ 2011; 93: 99–105. Article Google Scholar *
Broxmeyer HE, Lee MR, Hangoc G, Cooper S, Prasain N, Kim YJ _et al_. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial
progenitors from 21- to 23.5-year cryopreserved cord blood. _Blood_ 2011; 117: 4773–4777. Article CAS Google Scholar * Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM .
High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. _Proc Natl Acad Sci USA_ 2003; 100: 645–650. Article CAS
Google Scholar * Thyagarajan B, Berger M, Sumstad D, McKenna DH Jr . Loss of integrity of umbilical cord blood unit freezing bags: description and consequences. _Transfusion_ 2008; 48:
1138–1142. Article Google Scholar * Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR _et al_. Processing and cryopreservation of placental/umbilical cord
blood for unrelated bone marrow reconstitution. _Proc Natl Acad Sci USA_ 1995; 92: 10119–10122. Article CAS Google Scholar * Kaplan EL, Meier P . Nonparametric estimation from incomplete
observations. _J Amer Statist Assoc_ 1958; 53: 457–481. Article Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Stem Cell Transplantation and
Cellular Therapy, The University of Texas MD Anderson Cancer Center, Houston, TX, USA S Parmar, P Kongtim, A Alousi, C Hosing, U Popat, P Kebriaei, I McNiece, E Shpall, G Rondon & R
Champlin * University Hospitals Seidman Cancer Center, Case Western Reserve University, Cleveland, OH, USA M de Lima * Division of Pediatrics, The University of Texas MD Anderson Cancer
Center, Houston, TX, USA L Worth, D Petropoulos, D Lee & L Cooper Authors * S Parmar View author publications You can also search for this author inPubMed Google Scholar * M de Lima View
author publications You can also search for this author inPubMed Google Scholar * L Worth View author publications You can also search for this author inPubMed Google Scholar * D
Petropoulos View author publications You can also search for this author inPubMed Google Scholar * D Lee View author publications You can also search for this author inPubMed Google Scholar
* L Cooper View author publications You can also search for this author inPubMed Google Scholar * P Kongtim View author publications You can also search for this author inPubMed Google
Scholar * A Alousi View author publications You can also search for this author inPubMed Google Scholar * C Hosing View author publications You can also search for this author inPubMed
Google Scholar * U Popat View author publications You can also search for this author inPubMed Google Scholar * P Kebriaei View author publications You can also search for this author
inPubMed Google Scholar * I McNiece View author publications You can also search for this author inPubMed Google Scholar * E Shpall View author publications You can also search for this
author inPubMed Google Scholar * G Rondon View author publications You can also search for this author inPubMed Google Scholar * R Champlin View author publications You can also search for
this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to S Parmar. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Parmar, S., de Lima, M., Worth, L. _et al._ Is there an expiration date for a cord blood unit in storage?. _Bone
Marrow Transplant_ 49, 1109–1112 (2014). https://doi.org/10.1038/bmt.2014.92 Download citation * Published: 23 April 2014 * Issue Date: August 2014 * DOI: https://doi.org/10.1038/bmt.2014.92
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy
to clipboard Provided by the Springer Nature SharedIt content-sharing initiative