New frontiers in pediatric Allo-SCT: novel approaches for children and adolescents with ALL
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
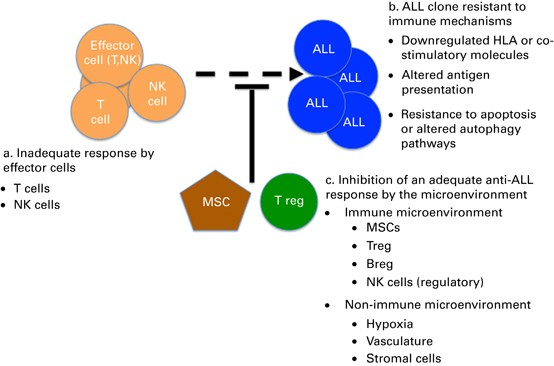
Although most children with ALL can be cured by chemotherapy approaches, allogeneic hematopoietic cell transplant (HCT) therapy offers a better chance of cure to selected high-risk patients
in first remission and most children who relapse. Although transplant-related mortality has decreased significantly in the past decade, relapse remains high after HCT for ALL; developing
strategies to decrease relapse and improve survival are vital. Recent studies have shown that relapse risk can be accurately defined using measurements of minimal residual disease (MRD) both
pre- and post-HCT and by knowing whether patients get GVHD in the first 2 months after transplant. With these risk definitions in hand, investigators are now applying novel agents and
immunotherapeutic methods in attempt to lower MRD before transplant and modulate the GVL effect after transplant. With powerful new immunological approaches coming on line, the transplant
process itself will likely expand to include pre and/or post-HCT interventions aimed at reducing relapse.
This study was supported in part by N01 HC-45220/HHSN268200425220C, U10 CA098543 and R01CA1116660. PBMTC activities were supported by 2U01HL069254 and the St Baldrick’s Foundation.
Division of Hematology and Hematological Malignancies, Primary Children’s Hospital, University of Utah School of Medicine/Huntsman Cancer Institute, Salt Lake City, UT, USA,
Division of Hematology, Oncology, and Blood and Marrow Transplantation, Children’s Center for Cancer and Blood Diseases, Children’s Hospital Los Angeles, The Norris Comprehensive Cancer
Center, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Department of Pediatrics, University of BC, BC Children's Hospital, Vancouver, British Columbia, Canada
ASW is a co-inventor on patents assigned to the United States Government, as represented by the Secretary, Department of Health and Human Services (National Institutes of Health), for some
of the investigational products reviewed in this paper. ASW also receives financial support through a Research Service Agreement between Children’s Hospital Los Angeles and MedImmune, LLC.
KRS is Vice-Chair of a protocol run through the Therapeutic Advances in Acute Leukemia Consortium that is sponsored by SBI Biotech. The remaining authors declare no conflict of interest.
Anyone you share the following link with will be able to read this content: