Allogeneic hematopoietic SCT for patients with autoimmune diseases
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Allogeneic hematopoietic SCT (HSCT) has been used as treatment for single patients with autoimmune diseases (AD). To summarise currently available information, we analyzed all patients who
underwent allogeneic HSCT for AD and who reported to the European Group for Blood and Marrow Transplantation (EBMT) database. Thirty-five patients receiving 38 allogeneic transplantations
for various hematological and non-hematological AD were identified. Four patients had had an allogeneic HSCT for a conventional hematological indication in the past. Fifty-five per cent of
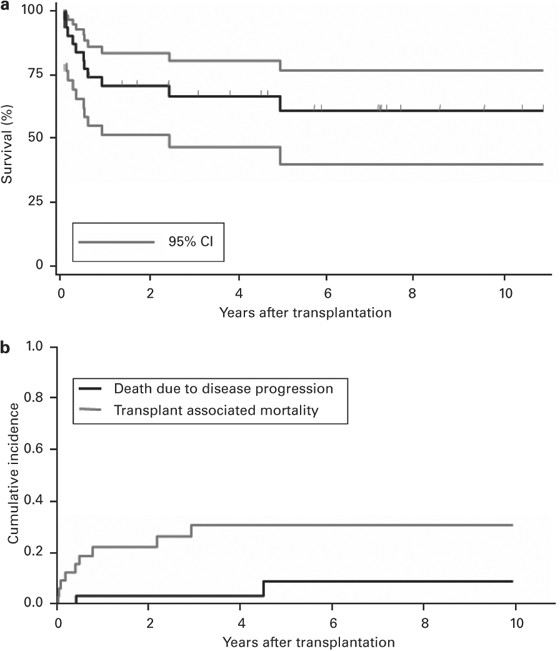
the transplantation procedures led to a complete clinical response of the refractory AD and 23% to at least a partial response. The median duration of response at the last follow-up was 70.7
(15.2–130) months. Three patients relapsed at a median of 12.3 months after HSCT. Treatment-related mortality at 2 years was 22.1% (95% CI: 7.3–36.9%). Two deaths were caused by progression
of AD. The probability of survival at 2 years was 70%. No single factor predicting the outcome could be identified. The retrospective nature of this study and the heterogeneous, partly
incomplete data are its limitations. However, allogeneic HSCT can induce remission in patients suffering from refractory AD. These data provide the basis for carefully conducted prospective
trials.
We thank Dr Martin Stern for help with statistics and for discussion of the paper. The cooperation of all participating teams and their staff (listed in the Appendix 1), the EBMT
Co-ordination office; Barcelona (F McDonald, E McGrath, SM Jones and EJ Mac Hale), Paris (V Chesnel, C Kenzey, C Durand and NC Gorin), London (C Ruiz de Elvira, S Hewerdine, S de Souza and N
Fortin-Robertson), the Austrian Registry (H Greinix, B Lindner), the Czech Registry (K Benesova, M Trnkova), the French Registry SFGM (D Blaise, C Raffoux and Z Chir), the German Registry
(H Ottinger, K Fuchs, C Müller, S Allgaier and A Müller), the Italian Registry (A Bacigalupo, R Oneto and B Bruno), the Dutch Registry (A Schattenberg, A v Biezen, M Sneets and R Brand), the
Spanish Registry (J Rifon, A Cedillo, and J López), the Swiss Registry (U Schanz, H Baldomero and E Buhrfeind), the Turkish Registry (G Gurman, M Arat, F Arpaci and M Ertem) and the British
Registry (C Craddock, J Cornish, K Towlson and M Wilson) is greatly appreciated. This study was supported in part by the European Leukemia Net LSH-2002-2.2.0-3, by a grant from the Swiss
National Research Foundation, 3200B0-118176, the Swiss Cancer League, the Regional Cancer League and the Horton Foundation. EBMT is supported by grants from the corporate members: Amgen
Europe GmbH, F Hoffmann-La Roche Ltd, Gilead Sciences UK, Miltenyl Biotec GmbH, Schering-Plough International Inc., Celegene International SARL, Genzyme, ViroPharma Europe, Chugai
Sanofi-Aventis SNC, Fresenius Biotech GmbH, Gambro BCT, Bayer Schering Pharma AG, Therakos, Bristol Myers Squibb, Novartis, Pharmacon, Cephalon, Pierre Fabre Médicament, GE Healthcare,
Alexion Europe, Pfizer, Biosafe SA, Merck Sharp and Dohme.
T Daikeler and T Hügle: These authors contributed equally to this work.
Department of Rheumatology, University of Basel, University Hospital, Basel, Switzerland
Department of Internal Medicine and INSERM U 697 Paris, Hôpital Saint-Louis, University Paris 7 Denis Diderot, Paris, France
Istituto per l'Infanzia ‘Burlo Garofolo’, Centro Trapianti Clinica Pediatrica Trieste, Trieste, Italy
Department of Hematology, San Martino Hospital, Genova, Italy
Department of Medicine, Hematology, University of Basel, University Hospital, Basel, Switzerland
Sahlgrenska University Hospital, Hematology Section, Goeteborg, Sweden
Hôpital Robert Debre, Haematology & Immunology Paediatric Unit, Paris, France
Universitätsklinikum Carl Gustav Carus, Klinik und Poliklinik für Kinder- und Jugendmedizin, Dresden, Germany
Department of Haematology, Royal Hospital for Sick Children, Glasgow, UK
Department of Internal Medicine, University Hospital, Innsbruck, Austria
Department of Oncology and Hematology, University Hospital, Strasbourg, France
Department of Hematology and Oncology, University Hospital, Innsbruck, Austria
Department of Medicine, Endocrinology, University of Basel, University Hospital, Basel, Switzerland
Musculoskeletal Research Group, Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, UK
Department of Clinical Immunology, Great Ormond Street Hospital London, London, UK
Hôpital Necker, Unité d'Immunologie et d'Hématologie and INSERM U 29, Paris, France.
Leiden University Hospital, BMT Centre Leiden, Leiden, The Netherlands.
University Medical Centre, Department of Haematology, Utrecht, The Netherlands.
Division of Stem Cell Transplantation and Immunotherapy, University Hospital Schleswig-Holstein, Kiel, Germany.
Hadassah University Hospital, Department of Bone Marrow Transplantation, Jerusalem, Israel.
Clinica di Oncoematologia Pediatrica, Dipartimento di Pediatria, Padova, Italy.
Charité-CVK, University Medicine Berlin, Pediatric Bone Marrow Transplant Service, Berlin, Germany.
Klinik/Poliklinik für Kinderheilkunde, Pädiatrische Hämatologie/Onkologie, Münster, Germany.
Unit of Hematology and Bone Marrow Transplantation, San Giovanni Rotondo, Italy.
Postgraduate Medical School, Department of Pediatrics/BMT Unit, Miskolc, Hungary.
University Hospital Gent, Haematology and Bloodbank, Gent, Belgium.
University Hospital of Palermo, Department of Hematology and Bone Marrow Transplantation, Palermo, Italy.
St László Hospital, Bone Marrow Transplantation Unit, Budapest, Hungary.
IRCCS Policlinico San Matteo, Pediatric Hematology–Oncology, Pavia, Italy.
Anyone you share the following link with will be able to read this content: