Construction kit for porous materials
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Monodisperse nanoparticles are used as building blocks in a new and versatile preparation of mesoporous structures. Mesoporous materials are usually made by templating methods or by the
assembly of molecular components into metal-organic frameworks. Applying these strategies to the synthesis of mesoporous metals, metal sulfides and selenides remains challenging, however, as
a result of their chemical reactivity and thermal behaviour. Now, Yadong Li and colleagues at Tsinghua University have made mesoporous structures with these compositions using the
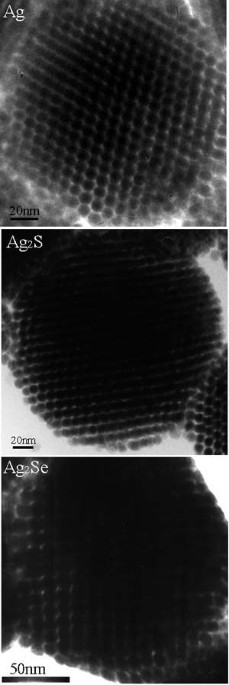
corresponding nanoparticles as structural building blocks.1 Fig. 1: A high resolution TEM image of Ag, AgS and AgSe colloidal spheres. To enable the fabrication of well-ordered porous
materials, the nanoparticles must be highly monodisperse. These uniform building blocks use surfactant technology to self-assemble into colloidal spheres with a 3-dimensional super-lattice
arrangement. Then, the spheres are calcined—heated to 300oC in an argon atmosphere—to remove the surfactants and form the mesoporous product. Li and colleagues demonstrated the method using
Ag, AgS and AgSe nanoparticles as starting materials, however, the advantage of their approach is its independence of the chemical composition of the particles. “If you are able to make
monodisperse nanocrystals of a material, then it should be possible to obtain the corresponding mesoporous products,” says Li. Indeed, the synthetic strategy was successfully adapted to make
mesoporous metal oxide materials from ‘artificial atoms’ such as NiO, MnO and ZnO.2 The Ag, AgS and AgSe building blocks can be made by a simple and efficient route which could be scaled up
with no detrimental effect to the product quality and yield. The size of the nanocrystals, which can be controlled by the reaction time, determines the pore size, and their monodispersity
determines the degree of order of the pores. Li and colleagues are now attempting to make mesoporous fluorides and phosphides. “In addition, we would like to tune the pore size from micro-
to meso- to macro, as well as synthesise hybrid mesoporous materials by mixing two or more kinds of crystals in the assembly process,” says Li. According to the Beijing group, the mesoporous
materials made by this new route show high porosity, good crystallization and thermal stability, as a result, they show promise in various applications including catalysis and lithium-ion
batteries. REFERENCES * Wang, D. S., Xie, T., Peng, Q., Li, Y. D. Ag, Ag2S, and Ag2Se Nanocrystals: Synthesis, Assembly, and Construction of Mesoporous Structures . _J. Am. Chem. Soc._ 130,
4016 – 4022 ( 2008 ). Article CAS Google Scholar * Wang, D. S., Xie, T., Peng, Q., Zhang, S. Y., Chen, J., Li, Y. D. _Chem. Eur. J._ 14, 2507 – 2513 ( 2008 ). Article CAS Google Scholar
Download references ADDITIONAL INFORMATION This research highlight has been approved by the author of the original article and all empirical data contained within has been provided by said
author. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Construction kit for porous materials. _NPG Asia Mater_ (2008).
https://doi.org/10.1038/asiamat.2008.52 Download citation * Published: 10 June 2008 * DOI: https://doi.org/10.1038/asiamat.2008.52 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative