Integration of a barley (hordeum vulgare) molecular linkage map with the position of genetic loci hosting 29 developmental mutants
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The first step in positional gene cloning is the integration into available molecular maps of genetic loci for which mutant alleles exist. We report the placement of 29 barley
developmental mutants on a restriction fragment length polymorphism–amplified fragment length polymorphism (RFLP–AFLP) map. The mapping procedure used homozygous mutant F2 plants in an
iterative process: once a mutant linked AFLP was found, primer combinations were successively selected to generate AFLP fragments more tightly linked to the mutant locus. The mutants
considered were _adp_, _als_, _aur-a1_, _aur-a2_, _br1_, _br2_, _bra-d7_, _cul3_, _cul5_, _cul15_, _cul16_, _den6_, _den8_, _dub1_, _hex-v3_, _hex-v4_, _int-c5_, _K_, _li, lig-a2_, _lk2_,
_lk5_, _sld1_, _sld4_, _tr_, _trd_, _unc_, _uc2_ and _uz_. The 29 mutant loci were linked to the closest molecular markers by distances ranging from 0 to 23 cM, with an average value of 3.8
cM away. Since the efficiency of the mapping procedure is a function of the density of molecular markers, the RFLP–AFLP map of Castiglioni _et al_ was further integrated with new AFLPs using
87 doubled haploid lines derived from the barley cross Igri × Danilo. A total of 819 mapped AFLP marker loci are now available in the combined map. SIMILAR CONTENT BEING VIEWED BY OTHERS
GRAS-DI SYSTEM FACILITATES HIGH-DENSITY GENETIC MAP CONSTRUCTION AND QTL IDENTIFICATION IN RECOMBINANT INBRED LINES OF THE WHEAT PROGENITOR _AEGILOPS TAUSCHII_ Article Open access 08
December 2020 CHROMOSOME GENOMICS FACILITATES THE MARKER DEVELOPMENT AND SELECTION OF WHEAT-_AEGILOPS BIUNCIALIS_ ADDITION, SUBSTITUTION AND TRANSLOCATION LINES Article Open access 22
November 2023 RECOMBINANT INBRED LINES AND NEXT-GENERATION SEQUENCING ENABLE RAPID IDENTIFICATION OF CANDIDATE GENES INVOLVED IN MORPHOLOGICAL AND AGRONOMIC TRAITS IN FOXTAIL MILLET Article
Open access 07 January 2022 INTRODUCTION Positional or map-based cloning techniques are widely used to identify genes defined by the existence of mutant alleles. In Arabidopsis, the
information generated by the Genome Initiative has demonstrated the value of this approach to discover new gene functions (Lukowitz et al, 2000). In crop species, progress in the enrichment
of molecular maps, as well as the availability of PCR-based markers and extensive collections of ESTs that facilitate SNP scanning, also favor the adoption of the positional cloning approach
(Richmond and Somerville, 2000). Barley has a genome size of 5 × 109 bp but, in spite of this size, positional cloning is feasible (Büschges et al, 1997; Lahaye et al, 1998; Schwarz et al,
1999). The first step in positional cloning is the localization, on the available molecular map, of the genetic loci defined by mutant alleles. We have developed a procedure for mapping
barley mutants and DNA probes based on amplified fragment length polymorphism (AFLP), in combination with restriction fragment length polymorphism (RFLP) (Castiglioni et al, 1998). The
strategy makes use of the Proctor × Nudinka (RFLP) map assembled by Heun et al (1991) and has proved to be reliable and efficient (Castiglioni et al, 1998; Pozzi et al, 2000; Schmitz et al,
2000; Müller et al, 2001). The positioning of mutant loci and expressed genes on the same linkage map allows the integration of map-based cloning procedures with the choice of candidate
genes. This approach has been successfully applied to the mapping of quantitative trait loci (QTLs) for disease resistance in _Capsicum_ and in wheat (Faris et al, 1999), while in maize a
candidate gene involved in flavone synthesis could be associated with resistance to corn earworm (Byrne et al, 1996). Further examples of the candidate gene approach for the cloning of
monogenic traits in plants are reported in Molinero-Rosales et al (1999) and Müller et al (1995). In this paper, we report on the enrichment of a barley molecular map by the addition of 368
AFLP loci and on the integration into this map of 29 genetic loci for which developmental mutants are described. The mutations investigated in this paper can be described in the light of an
interpretation of the morphology of the barley plant as a succession of growth units (phytomers; Bossinger et al, 1992). For the 29 mutants considered, growth units of a specific region of
the plant, which assume characteristics proper to phytomers of other regions, are evident for _uniculm_, _third outer_ _glume_, _densinodosum_, _intermedium_, _branched_ and _awned palea_.
Mutants characterized by the presence of modified organs on the unit of growth are _liguleless_, _bracteatum_, _triple awned lemma_ and _awned palea_. Mutants with additional phytomeres are
represented by _densinodosum_ and _branched_. MATERIALS AND METHODS PLANT MATERIAL A total of 87 doubled haploid barley lines (DH lines) were used to map 368 new AFLPs, which supplement the
511 already mapped by Castiglioni et al (1998). The 87 lines originated from a cross between the lines Igri and Danilo (courtesy of A Jahoor, Technische Universität München, Germany).
Developmental mutants were from the collection maintained at the Max-Planck-Institut für Züchtungsforschung (MPIZ), Cologne, Germany. This includes mutant stocks obtained from the Barley
Genetic Stock Center (BGS), Fort Collins, Colorado (at present Aberdeen, Idaho); the Istituto per la Cerealicoltura (FIOR), Fiorenzuola, Italy; and from Udda Lundqvist (UD), Svalöf
Collection, Svalöv, Sweden. The origins of mutants are listed in Castiglioni et al (1998). Mapping populations of the mutants listed in Table 1 were grown in the field and phenotypes
reclassified in greenhouse tests. Wild-type (WT) and mutant (M) plants were selected and stored as F3-seed families. Data concerning the sizes of the mapping populations are reported in
Table 1. Current mutant symbols for the loci described are reported in column 3 of Table 1 according to the recommendations of Lundqvist et al (1997), Franckowiak (1997), Franckowiak et al
(1997) and Franckowiak and Lundqvist (2002). In the following, the term ‘revised nomenclature’ refers to the gene symbols given in the four papers cited above. In Table 1, the symbols used
by Gustafsson et al (1969) are also reported, where applicable. DNA TECHNIQUES Seedlings were grown in the greenhouse and harvested at the four-leaf stage for DNA extraction. DNA was
extracted using the QIAGEN (Hilden, Germany) kit adapted to 96-well microtiter plates. The original AFLP procedure described by Zabeau and Vos (1993) was carried out with the minor
modifications specified by Castiglioni et al (1998). CONSTRUCTION OF THE MOLECULAR LINKAGE MAP USING DH LINES OF IGRI × DANILO The mapping data for the Proctor × Nudinka population were
integrated with a new set of AFLP bands mapped in an Igri × Danilo cross. _Eco_RI and _Mse_I AFLP primers were combined in the same 72 primer combinations used to develop the Proctor ×
Nudinka AFLP-RFLP map (Castiglioni et al, 1998). Each AFLP fragment was identified by the number of its primer combination and by an additional digit that refers to the figure stored as
‘Visual catalogue of AFLP bands polymorphic between the barley lines Igri and Danilo', at the web site http://www.diprove.unimi.it/. A list of the AFLP bands mapped on the Igri × Danilo
molecular linkage map is given at the same web site. In the 87 DH lines, polymorphic bands were scored as 0 or 1 for the absence or presence, respectively, and the data were tested for
conformity to a 1:1 segregation ratio using a _χ_2 test (_P_=0.05). Only bands whose segregation ratios did not deviate significantly from 1:1 were analyzed using the MAPMAKER program
(Lander et al, 1987; DOS version 3.0). Recombination data were at first analyzed at a LOD value of 10.0, allowing the definition of 28 preliminary linkage groups. Of those groups, 11 were
not positioned into the Nudinka × Proctor linkage groups since no bridge bands were shared by the Igri × Danilo and Nudinka × Proctor populations. On the remaining 17 preliminary linkage
groups, a three-point analysis was carried out and the ORDER routine of MAPMAKER was used to find the most probable order of their markers. An additional analysis at LOD 3.0 was performed on
each group to confirm marker order and allow the integration of previously unmapped markers into preliminary linkage groups. Finally, the ORDER procedure was used to allow placement of
markers previously not considered in the analysis and the TRY command allowed the placing of makers discarded by three-point analysis. Polymorphisms represented by segregating AFLP bands
were assigned to chromosomes and chromosomal subgroups based on bridge AFLP loci that segregated both in the Igri × Danilo and Proctor × Nudinka populations. Identity of AFLP bands produced
in the two populations by the same primer combination was assumed based on their electrophoretic mobility and relative intensity in gels (see also the Results and discussion). INTEGRATION OF
MUTANT LOCI INTO THE MOLECULAR MAP Linkage between mutants and markers was established based on DNA data from F3-seed families representing homozygous recessive F2 plants, as explained in
Pozzi et al (2000). The size of the mapping populations is reported in Table 1, together with the number of mapping populations produced. When linkage between an AFLP band and a given mutant
phenotype was established, the map position of the polymorphism was assigned based on the map of Castiglioni et al (1998), supplemented by the new Igri × Danilo data. Segregation data were
analyzed with the MAPMAKER program with LOD score value 3.0 and a maximum distance of 50 cM. The mutant locus was represented by a virtual marker in complete linkage to the mutant phenotype
in F2 plants. Coupling configurations – the presence of the AFLP band in the WT – were privileged because they allow the unambiguous classification of recombinants and, thus, a ready
detection of putative cases of linkage. In case of the absence of recombination between a mutant and a specific AFLP loci, and in order to verify the actual existence in the F2 population of
the concerned AFLP polymorphism, five to 10 WT F2 plants were also analyzed. RESULTS AND DISCUSSION THE IGRI × DANILO MAP The 87 DH lines derived from the Igri × Danilo cross were analyzed
with 72 AFLP primer combinations. Of 7668 readable bands (106.5 per primer combination), 520 were polymorphic and segregated in a ratio not significantly different from 1:1. Each primer
combination yielded, on average, 6.9 polymorphic AFLP bands. In total, 72 AFLP markers were selected as bridges because they represented bands that migrated at identical positions on AFLP
gels in both the Igri × Danilo and Proctor × Nudinka populations. Bridge bands were identified for 17 out of the 28 preliminary linkage groups and used to assign them to the seven linkage
groups of the Proctor × Nudinka population (Heun et al, 1991). The final Igri × Danilo map included 368 AFLP markers out of the 520 newly detected. The reduction was a consequence of the
presence of markers (1) placed on the 11 preliminary linkage groups that could not be assigned to a precise chromosomal location and (2) placed off-end by the ORDER command or that could not
be positioned by three-point analysis or by the TRY routine. The reduction in the number of usable markers also applied to bridge bands (from 72 to 61), mainly because of discrepancies in
their new map positions as compared with those derived from the Proctor × Nudinka map. This could be explained by the fact that while generating a large number of polymorphisms per gel, the
AFLP procedure produces a small fraction of bands with similar mobilities in gels and which map to different linkage groups. The final robustness of the new map, as obtained here for the
Igri × Danilo population, relies on the linkages of bridge bands with loci segregating only in the Igri × Danilo cross. Eventually, 16 bridge markers were allocated to Chromosome 7H; 11 to
Chromosome 2H; five to Chromosome 3H; eight to Chromosome 4H; six each to Chromosomes 1H and 6H and nine to Chromosome 5H. The order of, and the relative distance between, bridge markers in
the 17 chromosomal regions mapped in the cross Igri × Danilo were in agreement with the data of Castiglioni et al (1998). Data concerning the number of AFLP markers mapped on the Igri ×
Danilo linkage groups, the number of preliminary linkage groups assigned to each chromosome and the map length are reported in Table 2. A significant map expansion in comparison to the
previous RFLP–AFLP was observed, particularly in marker-dense regions (ie on Chromosomes 7H and 4H). Grouping of markers in specific regions of the seven linkage groups described in
Castiglioni et al (1998) identified, in that map, linkage subgroups. The same nomenclature was adopted to indicate linkage subgroups in the Igri × Danilo map, as described in the cited web
site. A graphic representation and statistics of the Igri × Danilo linkage map are obtainable at the web site http://www.diprove.unimi.it (PDF files and Table). MAPPING OF 29 BARLEY
DEVELOPMENTAL MUTANTS A procedure that allows rapid mapping to barley linkage groups of mutant alleles of genetically characterized loci is described in Castiglioni et al (1998). The
procedure uses homozygous recessive F2 plants segregating in crosses between the mutants and the two WT lines Proctor and Nudinka, the parents of the population used to create the reference
AFLP map. This procedure was used to map the loci _branched_ and _calcaroides_ (Castiglioni et al, 1998; Pozzi et al, 2000) and it is extended in this paper to a group of developmental
mutants. The mapping approach was iterative: once an AFLP polymorphism revealed linkage to a genetic locus, other primer combinations were selected to generate AFLP fragments which, based on
the Proctor × Nudinka and Igri × Danilo AFLP maps, were predicted to be in closer linkage to the mutant. The mapping data obtained are reported in Table 1. For each mutant, its map position
in the linkage subgroups of Castiglioni et al (1998) or in an interval spanning linkage subgroups, or in regions poor in polymorphic markers (the latter situation applied to two cases,
mutations _bra-d7_ and _unc_) is listed. For most mutants, F2 plants from the cross mutant × Nudinka proved to be sufficiently informative. In some cases, however, it was necessary to
reinforce the map position by using F2 plants from the Proctor cross (Table 1). In the case of the mutant _bra-d7_, the linked AFLP marker could not be localized on the Proctor × Nudinka
map, but the data available for the Igri × Danilo population made it possible to map it off-end on Chromosome 1H. The AFLP marker in closest linkage with the mutant locus, as determined in
coupling configuration on the largest mapping population analyzed, is indicated in Table 1 as the closest marker. The recombination distance between the closest marker and the next mutant
locus ranged from 0 to 23 cM, with an average of 3.8 cM. In about one-fourth of cases, the iterative procedure was performed until no recombinants were found between marker and mutant. In
Table 1, the number of markers within a linkage subgroup and linked to a given mutant is also reported. In few cases, only two markers were found linked to the mutant because of the absence
of polymorphisms for other markers of the region. The number of individuals included in the mapping populations was kept low to allow a rapid screening by AFLP: mapping populations ranged in
size from 16 to 72 F3 individuals (Table 1). In some cases, our data provide molecular markers for mutants previously assigned to chromosomes by linkage to other classical markers, thus
contributing linkage subgroups mapping. The mutants _adp_, _bra-d7_, _cul3_, _cul5_, _cul15_, _cul16_, _den6_, _den8_, _dub-1_, _sld4_ and _unc_ were here assigned to precise positions in
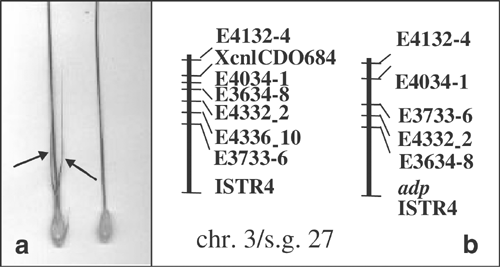
specific linkage groups for the first time. The mutant _awned palea_ (_adp_) was described by Ahokas (1977), and was mapped here for the first time to Chromosome 3H, linkage subgroup 27
(Figure 1). The _absent lower laterals_ (_als_) locus (Kasha and Walker, 1960; Tsuchiya and Haus, 1971) is here precisely mapped on Chromosome 3H, linkage subgroups 28 and 29. For this
locus, the revised nomenclature uses the same symbol. The mutant was induced with _γ_-rays in the variety Montcalm (Franckowiak, 1997). The mutations _auricleless_ (_aur-a1_ and _aur-a2_)
and _liguleless_ (_lig-a2_ and _li_), which affect ligule–auricle development, all mapped on Chromosome 2H, in an interval spanning sublinkage groups 21–23. The allele _li (lig)_, obtained
from BGS, a spontaneous mutant that is allelic to the Swedish _aur_ mutants (Tsuchiya, 1973a), is confirmed to map to Chromosome 2H as reported by Takahashi et al (1953). The mapping data
confirm allelism tests conducted by U Lundqvist (personal communication) in that _aur-a1_ and _aur-a2_ are allelic and both map on Chromosome 2H, linkage subgroups 20 and 21. The _lig-a2_
mutant is not allelic to _li_, according to U Lundqvist (personal communication), but both mutants comap with _aur_ mutations. The recommended nomenclature proposes also for _lig-a2_ the
symbol _lig_. Two _brachytic_ mutants _br_ and _br2_ (Franckowiak, 1995) were previously studied: _br1_ maps on Chromosome 7H, and is now assigned to sublinkage group 1 of the same
Chromosome. The mutant arose spontaneously in the variety Himalaya. The second mutant, _br2_, maps between sublinkage groups 38 and 40 on Chromosome 4H, confirming the chromosomal assignment
given by Tsuchiya (1980). _br2_ was induced by X-ray treatment in the variety Svanhals (Tsuchiya, 1962). The recommended nomenclature for these mutants is _brh_. The semibrachytic mutant
_uz_, obtained from BGS, is a spontaneous mutation present in several Japanese varieties. The mutant was previously assigned to Chromosome 3H (Takahashi and Yamamoto, 1951; Tsuchiya, 1980).
We mapped this mutant to sublinkage group 27 of Chromosome 3H. The symbol for the mutant locus in the recommended nomenclature is _uzu_. The _third outer glume_ (_trd_) mutants, also
referred to as _bracteatum_ (_bra_; Gustafsson et al, 1969), have been frequently investigated. The bract-like structures that in the mutant cover a triplet of spikelets, are homologous to a
normal leaf. Our data support the possibility that _bra-d7_ (which maps on Chromosome 1H south of sublinkage group 52), a mutant obtained from the Svalöf collection, and _trd_ (which maps
in the same chromosomal region and was obtained from the BGS) could be allelic. Tsuchiya (1974) previously proposed that _bra-c1_ and _trd_ were alleles. The mapping data support the
assignment by Konzak (1953) and Søgaard and von Wettstein-Knowles (1987) to Chromosome 1H. _bra-d7_ was obtained from Dr U Lundqvist (Svalöf collection) and _trd_ is the symbol used in the
recommended nomenclature. The _uniculm_ mutants (_uc_ in Søgaard and von Wettstein-Knowles, 1987) are characterized by a single culm and the absence of lateral tillers. The mutants we have
considered include a group obtained from Svalöf induced with X-rays and represented by _cul3_, _cul5, cul15_ and _cul16_, which all map on Chromosome 3H, sublinkage groups 31–33. The second
group includes the mutant _uc2_ obtained from BGS (Shands, 1963) and _unc_ isolated at MPIZ. Both mutants map on Chromosome 6H close to sublinkage group 54 in a region already identified for
_uc2_ by Robertson et al (1965). In the recommended nomenclature the two different loci hosting _uniculm_ mutants are designated _cul2_ and _cul4_ (Table 1). The two _densinodosum_ mutants
_den6_ and _den8_ originated from the Svalöf collection, but allelism tests were not available. Similar mutations (_multinoded branched, mnb_) were described by Walker et al (1963) and
(_multinoded dwarf, mnd_) by Prasad and Tripathi (1985), following the first description by Gustafsson (1947). Both _den6_ and _den8_ are now mapped on Chromosome 5H, sublinkage group 65.
The recommended nomenclature uses the symbol _mnd6_ for this locus. The mutant _double seed 1_ (_dub-1_) was obtained from the Svalöf collection and is here mapped to Chromosome 5H, linkage
subgroups 66 and 67. The two six-row ear character is controlled in barley by several independent gene loci, including the _v_ locus on Chromosome 2H (Nilan, 1964) – with the six-rowed
allele _v_ being inherited recessively – and the allelic series for the fertility of the lateral florets on Chromosome 4H (Gymer, 1977). A group of _intermedium_ genes has been described
that control the same character (Lundqvist, 1981; Lundqvist and Lundqvist, 1998): the _int-c5_ locus is assigned to Chromosome 4H (Woodward, 1949; Gymer, 1977). Based on our data, this locus
maps to the same chromosome, linkage subgroup 38. Most of the induced recessive mutants at the _v_ locus are designated by the symbol _hex-v_ (Gustafsson et al, 1969; Søgaard and von
Wettstein-Knowles, 1987). We have mapped two such alleles obtained from the Svalöf collection, _hex-v3_ and _hex-v4_, and they are, as expected, located on Chromosome 2H, linkage subgroups
19, 20. This locus is now indicated by the symbol _vrs1_. _Hooded_ (_K_) is a dominant mutant introduced into western countries from the Himalayas (Harlan, 1931; Stebbins and Yagil, 1966;
Badr et al, 2000). The mutation is caused by a tandem direct duplication on 305bp in the fourth intron of homeobox gene _Bkn_3, resulting in the ectopic overexpression of BKN3 at the
lemma–awn transition region: a local acquisition of meristematic activity is followed by the formation on the lemma of extra floral structures (Müller et al, 1995). The locus was assigned to
Chromosome 4H and now it has been associated to linkage subgroup 37 of the same linkage group. The recommended symbol is _Kap_. The _short awn_ (_lk_) mutants constitute a family of loci
that map to different chromosomes (Tsuchiya, 1973b). _lk5_ (Litzenberger and Green, 1951) was confirmed in this work to map on Chromosome 4H (Tsuchiya, 1980) linkage subgroup 38, while _lk2_
(Takahashi et al, 1953) lies on Chromosome 7H, linkage subgroup 6. The two loci now have the recommended symbols _lks5_ and _lks2_, respectively. The mutant _slender dwarf_ (_sld1_) was
induced by EMS treatment (Konishi, 1975; Szarejko and Maluszinski, 1984) and the gene was localized on Chromosome 3H near the mutant locus _uzu_ (Konishi et al, 1984). We have now refined
this map position by assigning the locus to linkage subgroup 27. The symbol for _sld1_ remains unchanged in the recommended nomenclature. _sld4_ obtained from FIOR was mapped here, for the
first time, to Chromosome 7H, linkage subgroup 6. The mutant _triple awned lemma_ (_tr_; Martini and Harlan, 1942) was here mapped to Chromosome 2H, linkage subgroups 22-23, in accordance
with Immer and Henderson (1943). The recommended symbol for the mutant is _trp_. The mapping activity reported is based on the use of AFLP markers, which allow one to score several
polymorphic loci in a single gel lane (Zabeau and Vos, 1993). When the goal is the construction of a molecular map of general value in genetic or plant breeding studies, the assumption has
to be made that cosegregating AFLP bands produced by amplifying DNAs of different genotypes derive from identical DNA sequences. This seems to be the case for barley (Waugh et al, 1997). Qi
et al (1998) and Qi and Lindhout (1996), for example, have produced an integrated AFLP map of barley linkage groups starting from four different segregating populations, using bridge AFLP
bands common to the populations considered and migrating at the same positions in AFLP gels. A recent study based on AFLP band sequencing indicates that, even in interspecific comparisons
within the genus _Hordeum_, AFLP product size and band intensity are reliable indicators of band DNA homology (El Rabey et al, 2002). It is therefore envisionable that AFLP polymorphisms
mapped in one population serve as chromosome-specific markers, if they also segregate in a second one. Some exceptions to this generalization have, however, been noted: in this paper, the
map positions of bridge markers agreed in general with published maps. Discrepancies noted could be due to coincidental comigration of AFLP fragments derived from distinct genomic positions.
Duplications of parts of the genome, as well as the existence in barley of a large genome fraction made up of repetitive sequences (Barakan et al, 1997), can account for the phenomenon.
This complication, in any case, did not interfere with the incorporation into the Proctor × Nudinka map of AFLP loci from the Igri × Danilo cross. Although the two populations have no
parents in common, and even if the parental lines themselves are not closely related, 61 common AFLP markers (17% of the total mapped) were available for the alignment of the two maps. The
linkage map obtained was in good agreement with previously published maps (Becker et al, 1995; Castiglioni et al, 1998; Qi et al, 1998 and references therein). This successful integration
makes now available a total of 819 AFLP marker loci useful for future mutant mapping and cloning in barley. The major contribution of this paper is the mapping data for 29 Mendelian mutants.
This allows to integrate these loci into a molecular map with an average density of about one AFLP marker every 3 cM. The average distance between the mapped genetic loci and the nearest
AFLP marker is 3.8 cM, but frequently no recombination events were detected. The small number of F2 plants analyzed may have contributed to reduce the probability of finding recombinants,
but local maker density frequently made also possible to select markers comapping with mutants. REFERENCES * Ahokas H (1977). A mutant of barley: _awned palea_. _Barley Genetics Newsletter_
7: 8–10. Google Scholar * Badr A, Müller K, Schaefer-Pregl R, El Rabey H, Effgen S, Ibrahim HH _et al_ (2000). On the origin and domestication history of barley (_Hordeum vulgare_). _Mol
Biol Evol_ 17: 499–510. Article CAS Google Scholar * Barakan A, Carels N, Bernardi G (1997). The distribution of genes in the genomes of Gramineae. _Proc Natl Acad Sci USA_ 94: 6857–6861.
Article Google Scholar * Becker J, Vos P, Kuiper M, Salamini F, Heun M (1995). Combined mapping of AFLP and RFLP markers in barley. _Mol Gen Genet_ 249: 65–73. Article CAS Google
Scholar * Bossinger G, Lundqvist U, Rohde W, Salamini F (1992). Genetics of plant development in barley. In: Munck L (ed) _Barley Genetics VI_, Munksgaard Intl. Publ. Ltd: Copenhagen,
Denmark. pp 989–1022. Google Scholar * Büschges R, Hollricher K, Panstruga R, Guus S, Wolter M, Frijters A _et al_ (1997). The barley _Mlo_ gene: a novel control element of plant pathogen
resistance. _Cell_ 88: 695–705. Article Google Scholar * Byrne PF, McMullen MD, Snook MD, Musket TA, Thuri JM, Widstrom NW _et al_ (1996). QTLs and metabolic pathways genetic control of
the concentration of maysin, a corn earworm resistance factor, in maze silks. _Proc Natl Acad Sci USA_ 93: 8820–8825. Article CAS Google Scholar * Castiglioni P, Pozzi C, Heun M, Terzi V,
Müller KJ, Rohde W _et al_ (1998). An AFLP-based procedure for the efficient mapping of mutations and DNA probes in barley. _Genetics_ 149: 2039–2056. CAS PubMed PubMed Central Google
Scholar * Dunford RP, Kurata N, Laurie DA, Money TA, Minobe Y, Moore G (1995). Conservation of fine-scale DNA marker order in the genomes of rice and the Triticeae. _Nucleic Acids Res_ 23:
2724–2728. Article CAS Google Scholar * El Rabey HA, Badr A, Schäfer-Pregl R, Martin B, Salamini F (2002). Species separation and incipient speciation in _Hordeum_ (Poaceae) resolved by
discontinuous molecular markers. _Plant Biol_ 4: 1–9. Article Google Scholar * Faris JD, Li WL, Liu DJ, Chen PD, Gill BS (1999). Candidate gene analysis of quantitative disease resistance
in wheat. _Theor Appl Genet_ 98: 219–225. Article CAS Google Scholar * Franckowiak J (1995). The brachytic class of semidwarf mutants in barley. _Barley Genetics Newsletter_ 24: 56.
Google Scholar * Franckowiak J (1997). Revised linkage maps for morphological markers in barley, _Hordeum_ _vulgare_. _Barley Genetics Newsletter_ 26: 9–21. Google Scholar * Franckowiak J,
Lundqvist U, Konishi T (1997). New revised names for barley genes. _Barley Genetics Newsletter_ 26: 4. Google Scholar * Franckowiak JD, Lundqvist U (2002). Descriptions of barley genetic
stocks for 2001. _Barley Genetics Newsletter_ 32: 49–137. Google Scholar * Gustafsson A (1947). Mutations in agricultural plants. _Hereditas_ 33: 1–100. Article Google Scholar *
Gustafsson A, Hagberg A, Lundqvist U, Persson G (1969). A proposed system of symbols for the collection of barley mutants at Svalof. _Hereditas_ 62: 409–414. Article Google Scholar * Gymer
PT (1977). Probable allelism of _Ii_ and _Int_-c genes. _Barley Genetics Newsletter_ 7: 34–35. Google Scholar * Harlan HV (1931). The origin of _Hooded_ barley. _J Hered_ 22: 265–272.
Article Google Scholar * Heun M, Kennedy E, Anderson JA, Lapitan NLV, Sorrels ME, Tanksley SD _et al_ (1991). Construction of a restriction fragment length polymorphism map for barley
(_Hordeum vulgare_). _Genome_ 34: 437–447. Article Google Scholar * Immer FR, Henderson MT (1943). Linkage studies in barley. _Genetics_ 28: 419–440. CAS PubMed PubMed Central Google
Scholar * Kasha KJ, Walker GWR (1960). Several recent barley mutants and their linkages. _Can J Genet Cytol_ 2: 397–415. Article Google Scholar * Konishi T (1975). Characteristics and
inheritance of EMS-induced mutants in barley. _Nogaku Kenkyu_ 55: 53–56. Google Scholar * Konishi T, Hayashi J, Moriya I, Takahashi R (1984). Inheritance and linkage studies in barley VII.
Location of six new genes on chromosome 3. _Ber Ohara Ins Landwirstch Forsch_ 18: 251–264. Google Scholar * Konzak CF (1953). The _third outer glume_ character in barley. _J Hered_ 44:
39–40. Article Google Scholar * Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE _et al_ (1987) MAPMAKER: an interactive computer package for constructing primary genetic
linkage maps of experimental and natural populations. _Genomics_ 1: 174–181. Article CAS Google Scholar * Lahaye T, Hartmann S, Topsch S, Freialdenhoven A, Yano M, Schulze-Lefert P
(1998). High-resolution genetic and physical mapping of the _Rar1_ locus in barley. _Theor Appl Genet_ 97: 526–534. Article CAS Google Scholar * Litzenberger SC, Green JM (1951).
Inheritance of awns in barley. _Agron J_ 43: 117–123. Article Google Scholar * Lukowitz W, Gillmor CS, Scheible W-R (2000). Positional cloning in Arabidopsis. Why it feels good to have a
genomic initiative working for you. _Plant Physiol_ 123: 795–805. Article CAS Google Scholar * Lundqvist U (1981). Intermedium _and_ hexastichon _mutants in barley_. Barley Genetics IV –
Proceedings of the IV International Barley Genetics Symposium, pp 908–912. * Lundqvist U, Lundqvist A (1998). Induced _intermedium_ mutants in barley: origin, morphology and inheritance.
_Hereditas_ 108: 13–26. Article Google Scholar * Lundqvist U, Franckowiak JD, Konishi T (1997). New and revised description of barley genes. _Barley Genetics Newsletter_ 26: 22. Google
Scholar * Martini ML, Harlan HV (1942). Barley Freaks. _J Hered_ 33: 338–343. Article Google Scholar * Molinero-Rosales N, Jamilena M, Zurita S, Gomez P, Capel J, Lozano R (1999).
FALSIFLORA, the tomato orthologue of FLORICAULA and LEAFY, controls flowering time and floral meristem identity. _Plant J_ 20: 685–693. Article CAS Google Scholar * Müller KJ, Romano N,
Gerstner O, Garcia-Maroto F, Pozzi C, Rohde W _et al_ (1995). The barley _Hooded_ mutation caused by a duplication in a homeobox gene intron. _Nature_ 374: 727–730. Article Google Scholar
* Müller J, Wang Y, Franzen R, Santi L, Salamini F, Rohde W . (2001). _In vitro_ interactions between barley TALE homeodomain proteins suggest a role for protein–protein associations in the
regulation of _Knox_ gene function. _Plant J_ 27: 13–23. Article Google Scholar * Nilan RA (1964). The cytology and genetics of barley 1951–1962. In: _Monographic Supplement, No. 3_,
Washington State University Press: Washington, DC. Vol 32, pp 1–278. Google Scholar * Pozzi C, Faccioli P, Terzi V, Stanca AM, Cerioli S, Castiglioni P _et al_ (2000). Genetics of mutation
affecting the development of a barley floral bract. _Genetics_ 154: 1335–1346. CAS PubMed PubMed Central Google Scholar * Prasad G, Tripathi DK (1985). Induced _multinoded_ mutants in
barley. _Barley Genetics Newsletter_ 15: 10–12. Google Scholar * Qi X, Lindhout P (1996). Comparison and integration of four barley genetic maps. _Genome_ 39: 379–394. Article CAS Google
Scholar * Qi X, Stam P, Lindhout P (1998). Use of locus-specific AFLP markers to construct a high-density molecular map in barley. _Theor Appl Genet_ 96: 376–384. Article CAS Google
Scholar * Richmond T, Somerville S (2000). Chasing the dream: plant EST microarrays. _Curr Opin Plant Biol_ 3: 108–116. Article CAS Google Scholar * Robertson DW, Wiebe GA, Shands RG,
Hagberg A (1965). A summary of linkage studies in cultivated barley, _Hordeum_ species: Supplement III, 1954–1963. _Crop Sci_ 5: 33–43. Article Google Scholar * Schmitz J, Franzen R,
Nguyen TH, Garcia-Maroto F, Pozzi C, Salamini F _et al_ (2000). Cloning, mapping and expression analysis of barley MADS-box genes. _Plant Mol Biol_ 42: 899–913. Article CAS Google Scholar
* Schwarz G, Michalek W, Mohler V, Wenzel G, Jahoor A (1999). Chromosome landing at the _Mla_ locus in barley (_Hordeum vulgare_ L.) by means of high-resolution mapping with AFLP markers.
_Theor Appl Genet_ 98: 521–530. Article CAS Google Scholar * Shands RG (1963). Inheritance and linkage of _orange lemma_ and _uniculm_ characters. _Barley Genetics Newsletter_ 6: 35–36.
Google Scholar * Søgaard B, von Wettstein-Knowles P (1987). Barley: genes and chromosomes. _Carlsberg Res Commun_ 52: 123–196. Article Google Scholar * Stebbins GL, Yagil E . (1966). The
morphogenetic effects of the _Hooded_ gene in barley. I. The course of development in _Hooded_ and _Awned_ genotypes. _Genetics_ 54: 727–741. CAS PubMed PubMed Central Google Scholar *
Szarejko I, Maluszinski M (1984). Two new dwarfism genes on barley chromosome 3. _Barley Genetics Newsletter_ 14: 35–38. Google Scholar * Takahashi R, Yamamoto J (1951). Studies on the
classification and geographical distribution of Japanese barley varieties. III. On the linkage relation and the origin of the _uzu_ or _semibrachytic_ character in barley. _Ber Ohara Inst
Landwirtsch Biol, Okayama Univ_ 9: 399–410. Google Scholar * Takahashi R, Yamamoto J, Yasuda S, Itano Y (1953). Inheritance and linkage studies in barley. _Ber Ohara Inst Landwirtsch Biol,
Okayama Univ_ 10: 29–52. Google Scholar * Tsuchiya T (1962). Radiation breeding in two-rowed barley. _Seiken Jiho_ 14: 21–34. Google Scholar * Tsuchiya T (1973a). Allelism testing between
established marker stocks and Swedish mutants. _Barley Genetics Newsletter_ 3: 67. Google Scholar * Tsuchiya T (1973b). New linkage maps of barley. _Barley Genetics Newsletter_ 3: 99–103.
Google Scholar * Tsuchiya T (1974). Further results of allelism testing in barley. _Barley Genetics Newsletter_ 4: 82–85. Google Scholar * Tsuchiya T (1980). Description of genetics
stocks. _Barley Genetics Newsletter_ 10: 108–119. Google Scholar * Tsuchiya T, Haus TE (1971). Absent lower laterals. _Barley Genetics Newsletter_ 1: 123. Google Scholar * Walker GW,
Dietrich J, Miller R, Kasha K (1963). Recent barley mutants and their linkages. II. Genetic data for further mutants. _Can J Genet Cytol_ 5: 200–219. Article Google Scholar * Waugh R,
Bonar N, Baird E, Thomas B, Graner A, Hayes P _et al_ (1997). Homology of AFLP products in three mapping populations of barley. _Mol Gen Genet_ 255: 311–321. Article CAS Google Scholar *
Williams RF (1975). _The Shoot Apex and Leaf Growth_, Cambridge University Press: Cambridge. Book Google Scholar * Woodward RW (1949). The inheritance of fertility in the lateral florets
of the four barley groups. _Agron J_ 41: 317–322. Article Google Scholar * Zabeau M, Vos P (1993). Selective restriction fragment amplification: a general method for DNA fingerprinting.
_European Patent Application_ no. 92402629.7. Download references ACKNOWLEDGEMENTS This work was funded by the EGRAM-EuDicotMap Project of the European Community. AUTHOR INFORMATION Author
notes * C Pozzi Present address: Fondazione Parco Tecnologico Padano-CERSA, c/o DiProVe, University of Milano, 20133, Milano, Italy AUTHORS AND AFFILIATIONS * Max-Planck-Institut für
Züchtungsforschung, Carl-von-Linné -Weg, Cologne, 10 50829, Germany C Pozzi, D di Pietro, G Halas, C Roig & F Salamini Authors * C Pozzi View author publications You can also search for
this author inPubMed Google Scholar * D di Pietro View author publications You can also search for this author inPubMed Google Scholar * G Halas View author publications You can also search
for this author inPubMed Google Scholar * C Roig View author publications You can also search for this author inPubMed Google Scholar * F Salamini View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to F Salamini. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pozzi,
C., di Pietro, D., Halas, G. _et al._ Integration of a barley (_Hordeum vulgare_) molecular linkage map with the position of genetic loci hosting 29 developmental mutants. _Heredity_ 90,
390–396 (2003). https://doi.org/10.1038/sj.hdy.6800259 Download citation * Received: 18 October 2002 * Accepted: 17 January 2003 * Published: 25 April 2003 * Issue Date: 01 May 2003 * DOI:
https://doi.org/10.1038/sj.hdy.6800259 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * linkage map * mutant mapping * AFLP