A method for true endothelial cell (tencell) transplantation using a custom-made cannula for the treatment of endothelial cell failure
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT AIM To describe a novel technique, true endothelial cell (Tencell) transplant, for the transfer of donor endothelial cells with only a Descemet's carrier in patients with
endothelial cell failure. METHOD Three patients treated for endothelial cell failure underwent Tencell transplantation. Two were performed to alleviate pain from bullous keratopathy, and one
was performed to improve vision. Preoperative pain, vision, and corneal thickness were recorded and compared to the same parameters postoperatively. RESULTS At 3 months postoperatively, all
patients were pain free. The visual acuity had improved in all cases, and all three cases demonstrated a reduction of central corneal thickness. In two cases it was possible to perform an
endothelial cell count. CONCLUSION This is the first description of Tencell transplantation in living subjects. SIMILAR CONTENT BEING VIEWED BY OTHERS RISK FACTORS FOR ENDOTHELIAL CELL LOSS
AFTER DESCEMET MEMBRANE ENDOTHELIAL KERATOPLASTY (DMEK) Article Open access 06 July 2020 CULTIVATED AUTOLOGOUS LIMBAL EPITHELIAL CELL (CALEC) TRANSPLANTATION FOR LIMBAL STEM CELL DEFICIENCY:
A PHASE I/II CLINICAL TRIAL OF THE FIRST XENOBIOTIC-FREE, SERUM-FREE, ANTIBIOTIC-FREE MANUFACTURING PROTOCOL DEVELOPED IN THE US Article Open access 04 March 2025 CLINICAL OUTCOME ANALYSIS
OF TWO APPROACHES TO TRYPAN BLUE DYEING FOR DMEK Article Open access 19 July 2024 INTRODUCTION Endothelial cell failure is one of the most common reasons for penetrating keratoplasty (PK).
However, the architectural disruption caused by PK can induce high degrees of both regular and irregular astigmatism and is often associated with a prolonged postoperative recovery. Recent
developments in deep lamellar endothelial keratoplasty (DLEK) lessen corneal disruption, so reducing induced astigmatism and enabling a more rapid visual recovery.1, 2, 3 However, the
technique of DLEK is technically demanding. Additionally, it involves creating a stroma-to-stroma interface, which can reduce the final best-corrected visual acuity. Transplantation of
Descemet's membrane carrying viable endothelium has been described in human cadaver eyes.4 This has not, as yet, become a popular technique, perhaps owing to difficulties in harvesting
the donor endothelial layer and its application to the host inner corneal surface without viable endothelial cell loss. A technique that achieves the transfer of a disc of donor endothelial
cells with only a Descemet's carrier, onto the posterior surface of the cornea, quickly and effectively via an 8.00 mm corneal incision is described. MATERIALS AND METHODS Three cases
are described. All had endothelial cell failure and all underwent Tencell transplant. Fully informed consent was obtained for all patients prior to surgery. All patients were made aware that
there were several surgical options available to treat their condition. They were all aware that this technique was new and unproven. Both pre- and postoperative Snellen visual acuity,
central corneal thickness, and endothelial cell counts were measured. Central corneal thickness was measured from an average of three consecutive recordings using an ultrasound pachymeter
(Quantel Pocket). Endothelial cell counts were measured, where possible, using a Topcon SP100 cell counter. A single reading was taken from the central cornea. SURGICAL TECHNIQUE The
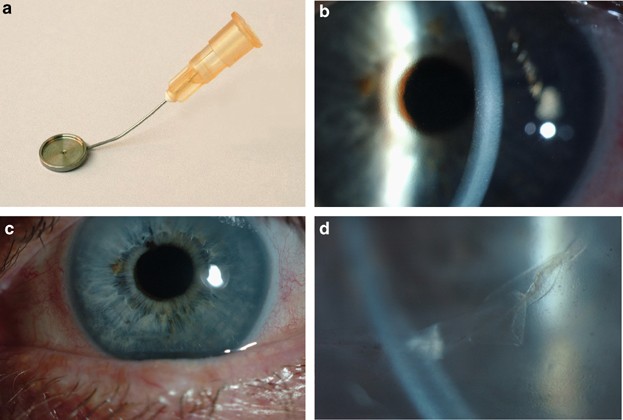
technique uses a specifically designed instrument (Altomed) (Figure 1a), which is a cannula that ends in a flat platform at its distal end to support the endothelial cell layer. The platform
facilitates transfer of the disc of Descemet's membrane and endothelium into the anterior chamber. The lumen of the cannula opens in the centre of this platform and is attached to a
2.5 ml syringe at its proximal end. Injection of air floats the donor disc up to adhere to host corneal stroma. PREPARATION OF THE RECIPIENT Preparation of the recipient was performed before
donor preparation to prevent undesired drying or damage to the endothelial cell layer. The affected eye was dilated with guttae tropicamide 1% (Chauvin). An 8.0 mm partial thickness
superior corneal or limbal incision was made using a 15° blade (Alcon Surgical), of approximately 2/3 corneal depth, similar to that for an extracapsular cataract extraction. A 27-G needle
was bent at the tip by 30° so that it could be introduced into the anterior chamber, with the point bent upwards to perform the descemetorhexis.5 A 27-G needle was used, as it created its
own self-sealing entry port in the peripheral cornea. The anterior chamber could therefore be maintained without the use of an anterior chamber maintainer or viscoelastic. Viscoelastic was
not used to avoid the risk of any residual coating impairing adhesion of the endothelial cell layer. The visualisation of the descemetorhexis was facilitated using the red reflex. A central
area of Descemet's membrane, approximately 7.5 mm in diameter, was removed. The operculum of Descemet's membrane was then aspirated via the paracentesis using a Simco cannula.
There was no other preparation of the recipient cornea such as roughening of the stroma or stab incisions to permit fluid release. The anterior chamber was left formed with balanced salt
solution (BSS Alcon laboratories). PREPARATION OF THE DONOR MATERIAL The donor sclerocorneal button was placed epithelial side down on a silicone block corneal holder (Altomed). A shallow
trephination of the endothelial surface was performed using a 7.5 mm long-handled trephine (Altomed). The edge of the circular disc of Descemet's membrane was held with two plane-tipped
micro-forceps. This was gently peeled from the stroma and placed endothelial side down on a pre-prepared cannula, which had a layer of hydroxymethylcellulose on its carrier surface to
protect the endothelial cells, and was mounted on a 2.5 ml syringe filled with air. The partial thickness incision in the host eye was then made full thickness, thus creating a two-step
corneal incision. The donor disc was introduced into the anterior chamber using Tappin's cannula. Once in the anterior chamber, the spatula was elevated and air was injected from the
syringe through the centre of the cannula, which separated the donor disc from the spatula and apposed the endothelial disc to the bare stroma. The spatula was then removed and the anterior
chamber was filled completely with air. The 8.0 mm section was sutured with five 10/0 nylon sutures. The anterior chamber was completely filled with air and left for approximately 5 min. The
air bubble was then reduced to a mobile bubble, half the diameter of the cornea. The patient was positioned in a supine position for half an hour postoperatively. Subconjunctival injections
of cefuroxime 125 mg and betnesol 4 mg were administered at the end of the procedure. Postoperative guttae chloramphenicol and guttae dexamethasone 0.1% were used four times a day for the
first week. Guttae dexamethasone was then continued four times a day for the next 6 months, in a regimen similar to that following a PK. CASE 1 An 86-year-old female had undergone left
extracapsular cataract extraction (ECCE) complicated by vitreous loss in 1989 and had a sutured posterior chamber lens in 2003. Postoperative visual acuity had been 6/18 with a refraction of
+1.50/−7.00 at 96°, but the patient had subsequently developed age-related macular degeneration and then bullous keratopathy. The vision dropped to counting fingers at 1 m and the cornea
was oedematous and thickened (pachymetry: 670 _μ_m). There was no inflammation and the intraocular pressure was normal. The cornea was too oedematous to permit a preoperative endothelial
cell count. CASE 2 A 68-year-old man with Fuchs endothelial dystrophy had undergone routine small-incision cataract extraction with foldable intraocular lens implantation. Unfortunately, his
vision did not improve to an acceptable level. In the early postoperative period, the visual recovery was impeded by the development of cystoid macula oedema (CMO). Visual rehabilitation
was complicated by corneal oedema but no bullous keratopathy, and this was still present at 6 months. There was considerable glare and best-corrected visual acuity was 6/12. The cornea was
too oedematous to permit a preoperative endothelial cell count. The central corneal thickness was 647 _μ_m, with obvious guttata (Figure 1b). The patient agreed to undergo a Tencell
transplant. Unfortunately, during the procedure, the donor endothelium tore and the procedure was abandoned. It was repeated 2 weeks later and proceeded uneventfully. CASE 3 A 65-year-old
Indian lady presented with a painful left eye. The vision was perception of light. She had corneal decompensation with bullous keratopathy and a central corneal thickness of 593 _μ_m. There
was also a pupillary membrane, which prevented any view of the fundus. Initially she was given a bandage contact lens to alleviate the pain, but she was keen for an attempt to improve vision
further. She underwent a combined procedure involving removal of the pupillary membrane, extracapsular cataract extraction with lens implantation, and endothelial cell transplant. RESULTS
CASE 1 At 1 month postoperatively, there was a central area of clear cornea and the vision had improved from CF to 6/36. The vision remained 6/36 at the 3-month review; however, peripheral
corneal epithelial oedema remained. The central endothelial cell count was 377 cells per mm2. CASE 2 Postoperative recovery was initially unremarkable. At 1 week, the vision was 6/36 and he
felt the vision was improving. Unfortunately, 4 weeks after the Tencell transplant, CMO developed and the vision failed to improve further. Treatment was with guttae ketorolac trometamol
three times a day, dexamethasone 0.1% four times a day, and an orbital floor injection of aqueous methylprednisolone acetate 40 mg. The CMO resolved. At 3 months after the Tencell
transplant, visual acuity was 6/9. The central cornea was clear (Figure 1c) and the endothelial cell count was 1100 cells/mm2. There was clear demarcation between the central corneal region,
which was clear and markedly thinner, and the peripheral, thicker, cornea. At the level of the endothelium there was a rolled edge where the donor endothelium came into contact with the
loose edge of the recipient endothelium (Figure 1d, Table 1). CASE 3 At 2 months postoperatively, visual acuity had improved from PL to 6/60. The cornea was clearer with no bullae, and she
was pain free. Scarring of the cornea limited further visual improvement. At the 3-month review the vision remained 6/60. The pachymetry measurements of the central cornea were not very
thick preoperatively despite the presence of bullous keratopathy, and reflected either corneal scarring or an unusually thin cornea. DISCUSSION This novel technique describes the use of a
purpose-made cannula to transfer an endothelial disc to bare host corneal stroma, elevated to position, without wrinkles, using an injected air bubble. The first and third cases had a
guarded visual prognosis and were performed to relieve bullous keratopathy. The endothelial cell transplant in these cases was considered to be successful, as indicated by the lack of
epithelial oedema and lack of pain. All three cases had thinner pachymetry postoperatively, indicating the successful transplantation of viable endothelial cells. The first case had a very
low endothelial cell count postoperatively. Despite this, the central cornea was clear and a cell count could be recorded, which had not been possible preoperatively on account of gross
corneal oedema. However, the high cell loss indicates a need to improve the harvesting or transfer techniques in order to reduce traumatic endothelial cell loss. The second case encountered
several problems. Firstly, a torn endothelial layer, which meant that the procedure had to be repeated 2 weeks later. It does, however, demonstrate that the procedure can be attempted on
more than one occasion, a few weeks apart, with a successful result. It may have been advantageous to have prepared the donor material before the recipient to prevent this complication.
Secondly, the patient developed CMO, a month after the Tencell transplant. The diagnosis was made clinically and was evidence of a clearing cornea. The CMO settled on treatment. It remains
to be seen whether the incidence of CMO is higher with this technique. However, this patient developed CMO after his initial cataract extraction and it is unlikely that CMO is specifically
associated with this technique. Tencell transplantation is a quick and potentially simple method to treat endothelial cell failure. Like DLEK, it provides a rapid visual recovery from
corneal decompensation, a condition that has traditionally been managed by PK. Architectural disruption of the cornea has limited the success of PK owing to high degrees of induced regular
and irregular astigmatism, ametropia, prolonged healing, and fluctuating refractive results. The development of DLEK has demonstrated the ability to transplant endothelium and preserve the
surface architecture of the cornea, enabling a more rapid recovery with a more stable and predictable postoperative refraction. DLEK involves a posterior lamellar dissection of both donor
and recipient corneal stroma. The failed host endothelium and posterior stroma are then replaced with a button of donor endothelium on a thin lamella of donor stroma. Tencell transplantation
avoids the manual lamellar dissection of both the donor and recipient stroma and the complications of converting to PK6 if there is inadvertent perforation of the recipient cornea during
dissection. Our personal experience with DLEK is that there can be problems with adhesion of the donor button to the recipient stroma. Selective endothelial cell replacement with only a
Descemet's membrane carrier may potentially be a way of avoiding this problem. The low postoperative endothelial cell counts are probably because of trauma during the harvesting and
transfer process. This needs improvement to match the excellent cell counts from DLEK.6 A larger series is needed to assess the long-term survival of the endothelial cells and the long-term
visual outcomes using this method. REFERENCES * Melles GRJ, Lander F, van Dooren BTH, Pels E, Houdijn Beekhuis W . Preliminary clinical results of posterior lamellar keratoplasty through a
sclerocorneal pocket incision. _Ophthalmology_ 2000; 107: 1850–1857. Article CAS Google Scholar * Terry MA . A new approach for endothelial transplantation; deep lamellar endothalial
keratoplasty. _Int Ophthal Clin_ 2003; 43(3): 183–193. Article Google Scholar * Melles GRJ, Lander F, Nieuwendaal C . Sutureless, posterior lamellar keratoplasty. _Cornea_ 2002; 21(3):
325–327. Article Google Scholar * Melles GRJ, Lander F, Rietveld F . Transplantation of Descemet's membrane carrying viable endothelium through a small scleral incision. _Cornea_
2002; 21(4): 415–418. Article Google Scholar * Melles GRJ, Wijdh RHJ, Nieuwendaal MD . A technique to excise the descemet membrane from a recipient cornea (Descemetorhexis). _Cornea_ 2004;
23(3): 286–288. Article Google Scholar * Ousley PJ, Terry MA . Stability of vision, topography, and cell density from 1 year to 2 years after deep lamellar endothelial keratoplasty
surgery. _Ophthalmology_ 2005; 112: 50–57. Article Google Scholar Download references ACKNOWLEDGEMENTS I thank my wife for her help in the preparation of this manuscript. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Ophthalmology Department, Ashford and St Peter's NHS Trust, Guildford, UK M Tappin Authors * M Tappin View author publications You can also search
for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to M Tappin. ADDITIONAL INFORMATION Conflict of interest: none No grants received No proprietary interest RIGHTS
AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tappin, M. A method for true endothelial cell (Tencell) transplantation using a custom-made cannula for the
treatment of endothelial cell failure. _Eye_ 21, 775–779 (2007). https://doi.org/10.1038/sj.eye.6702326 Download citation * Received: 02 September 2005 * Accepted: 29 January 2006 *
Published: 31 March 2006 * Issue Date: 01 June 2007 * DOI: https://doi.org/10.1038/sj.eye.6702326 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
KEYWORDS * endothelial dystrophy * endothelial transplant * corneal graft