Cardiolipin and phosphatidylglycerol are not required for the in vivo action of Bcl-2 family proteins
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
The release of the cytochrome c (cyt. c) and several other protein factors from the mitochondrial intermembrane space results in the activation of caspases, proteases responsible for cell
destruction during programmed cell death (PCD) and is considered an initiation and commitment point in the apoptotic pathway. Release of these factors from the mitochondria, in response to a
broad range of signals or insults, is regulated by proteins in the Bcl-2 family.1, 2 This family consists of both proapoptotic proteins (e.g. Bax, Bak, Bid) that promote the release of
mitochondrial factors and antiapoptotic proteins (e.g. Bcl-XL and Bcl-2) that prevent the release of these factors in the absence of death signal. Proteins in this family share up to four
homologous BH1-4 (Bcl-homology) domains and many of them also have C-terminal hydrophobic domain that targets these proteins to membranes. A subset of proapoptotic proteins (e.g. Bid) that
contain only BH3 domain (BH3-only proteins) are located in various cellular compartments where they can be released in response to a variety of death signals, allowing them to transfer the
signal to the multidomain proapoptotic proteins Bax and Bak. When activated, in part, by interaction with BH3-only proteins, both Bax and Bak oligomerize in the outer mitochondrial membrane.
In vitro studies support the idea that this oligomerization results in the formation of an aqueous pore of sufficient size to facilitate translocation of cyt. c, and perhaps other
components of the mitochondrial intermembrane space, into the cytosol where they then initiate and modulate the apoptotic cascade.3, 4, 5
The precise molecular mechanisms by which multidomain proapoptotic proteins like Bax and Bak are activated by BH3-only proteins have not yet been completely resolved. Additionally, it is
also not clear whether pore formation forms the basis of the action of these proteins during PCD in vivo and whether pore formation in vivo requires additional components or is based on the
action of these proapoptotic proteins alone. Several studies have suggested that the process of pore formation requires, or is facilitated by, the interaction of Bax (or Bak) with a yet to
be identified cytosolic6 or mitochondrial protein.7 Cardiolipin (CL), a unique phospholipid of the inner mitochondrial membrane, has also been recently proposed to be required in the action
of proapoptotic Bcl-2 proteins.8, 9 These in vitro studies have suggested that CL is required for the Bax-mediated permeabilization of phospholipid vesicles; Bax-mediated permeabilization
does not proceed in the absence of CL.9 In contrast, others10 have shown that recombinant Bax is able to induce release of cyt. c from the mitochondria lacking CL following isolation of
these organelles from yeast strains in which the gene encoding for cardiolipin synthase (CL synthase; CRD1),11 required for CL synthesis, had been eliminated (Δcrd1 strains). However,
phosphatidylglycerol (PG), the intermediate in the synthesis of the CL, accumulates in the mitochondrial membranes of Δcrd1 strains and is known to be able to substitute functionally for the
CL.12, 13 Thus, it is not yet clear whether CL or, in the absence of CL, PG is required for the in vivo mitochondrial action of proapoptotic molecules like Bax.
To test directly the requirement of either CL or PG in the in vivo action of multidomain, proapoptotic members of the Bcl-2 family, we have assessed the response of yeast (Saccharomyces
cerevisiae) lacking genes required for the synthesis of both CL and PG to the expression of Bax and Bcl-XL. Although the yeast genome does not encode Bcl-2 family members or other proteins
involved in PCD in metazoan cells, expression of proapoptotic Bcl-2 family members like Bax results in yeast cell death.14, 15, 16 Once expressed, Bax is constitutively targeted to the outer
mitochondrial membrane where it mediates alterations in mitochondrial function similar to those observed in mammalian cells: release of cyt. c, mitochondrial swelling, alterations of
mitochondrial membrane potential, mitochondrial matrix alkalinization and cytosolic acidification.14, 17, 18, 19 In contrast, antiapoptotic Bcl-2 family members like Bcl-2 and Bcl-XL
suppress Bax-induced cell death when coexpressed in yeast and are also targeted to the outer mitochondrial membrane.16, 20, 21 All results generated in these studies employing yeast strongly
indicate that Bcl-2 family members act directly upon highly conserved mitochondrial components that correspond directly to their apoptotic substrates in mammalian cells, and furthermore
that all cellular components necessary for the formation of pore by Bax are highly conserved and present in yeast mitochondria. Thus, the availability of yeast as an alternative system in
which to study the action of the proteins of the Bcl-2 family together with the powerful techniques of yeast genetics has made it possible to test the participation of various cellular
components, such as the voltage-dependent anion channel and ATP/ADP translocator, in the process of the permeabilization of the mitochondrial membrane by Bcl-2 family members.18, 22, 23, 24
To test whether proapoptotic proteins like Bax require CL to kill cells in vivo, we examined Bax activity in yeast cells devoid of CL due to deletion of CRD1, the yeast gene encoding CL
synthase that is required for the biosynthesis of this unique mitochondrial phospholipid.11 CRD1 was eliminated by standard gene transplacement methods. CRD1-deficient strains (Δcrd1)
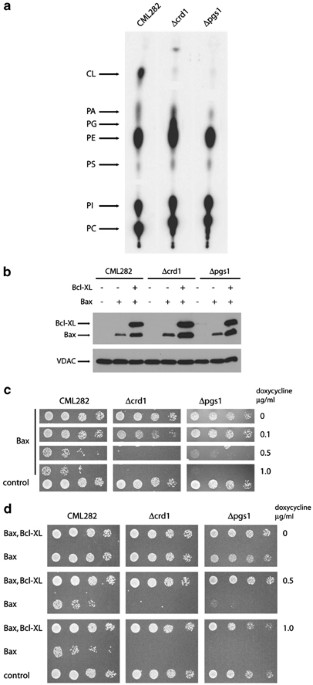
contained no detectable CL (Figure 1a). However, as had been noted in previous studies, Δcrd1 strains contained elevated level of PG, a biosynthetic precursor of CL (Figure 1a and Chang et
al.11). These earlier studies also demonstrated that PG is able to compensate partially for the absence of CL.12, 13 Consequently, we also generated CML282 derivatives in which PGS1, the
gene encoding PG-P synthase,12 has been inactivated. As expected, PGS1-deficient strains (Δpgs1) contained no detectable CL or PG (Figure 1a). Low amounts of radiolabel at the positions of
PG and CL do not migrate at positions of authentic PG or CL in two-dimensional systems (not shown). As expected, Δpgs1 strains were also respiratory incompetent (not shown).12
CRD1 and PGS1 genes were inactivated in the yeast strain CML282 by the standard gene transplacement technique. (a) Phospholipid analysis of mutant strains: Wild-type (CML282), Δcrd1 and
Δpgs1 cells were grown overnight (at least six generations) in synthetic complete medium with [32P]Pi. Lipids were extracted as described earlier29 and separated by thin-layer chromatography
using chloroform : methanol : acetic acid (65 : 28 : 8) as the solvent system. The mobility of CL, phosphatidic acid (PA), phosphatidylglycerol (PG), phosphatidylethanolamine (PE),
phosphatidylserine (PS), phosphatidylinositol (PI) and phosphatidylcholine (PC) are indicated. (b) Expression of Bax and Bcl-XL in wild-type, Δcrd1 and Δpgs1 strains: Wild-type, Δcrd1 and
Δpgs1 cells were cotransformed with vector controls, pCM252-U-HA-Bax, or pCM252-HA-Bcl-XL and pCM252-U-HA-Bax and grown overnight in glucose synthetic complete media, cells were harvested,
washed and diluted into synthetic complete media with 2% raffinose and 1 μg/ml doxycycline. Cells were then grown for 24 h. Whole-cell protein extracts were separated by a 12.5% PAGE,
transferred on a nitrocellulose membrane and probed with anti-HA and anti-yVDAC1 antibodies. The apparent difference in expression levels in cells transformed with HA-Bax expression plasmids
versus those transformed with both HA-Bax and HA-Bcl-XL expression plasmids reflects the fact that expression of HA-Bax alone leads to cell death, while expression of both HA-Bax and
HA-Bcl-XL permits cell growth in response to the addition of doxycycline. (c) Response of Δcrd1 and Δpgs1 yeast strains to the expression of Bax: Wild-type, Δcrd1 and Δpgs1 cells transformed
with vectors or pCM252-U-HA-Bax expression plasmids were grown in liquid synthetic complete media and five-fold serial dilutions of cell suspensions were spotted onto plates containing 2%
raffinose and indicated concentration of doxycycline. The growth was assessed after 3 to 5 days. (d) Expression of Bcl-XL rescues mutant strains from Bax-mediated killing: Wild-type, Δcrd1
and Δpgs1 cells cotransformed with vectors or pCM252-U-HA-Bax and pCM252-HA-BclXL were grown in liquid synthetic complete media and five-fold serial dilutions of cell suspensions were
spotted onto plates containing 2% raffinose and indicated concentration of doxycycline. The growth was assessed after 3 to 5 days
To test the ability of Bax to kill cells lacking CL and PG, we took advantage of the fact that CML282 constitutively expresses a modified tetR protein that mediates the expression of target
genes placed downstream of tetO sequences in response to tetracycline or its analogs (reverse or ‘tet-on’ system). Earlier studies had demonstrated that the level of Bax expression under
these conditions is proportional to the concentration of doxycycline present in the media; higher concentrations lead to higher levels of Bax expression.25 Accordingly, Δcrd1, Δpgs1 and
wild-type cells were transformed with plasmid-containing sequences encoding an N-terminally, HA-tagged Bax protein downstream of the Tet-inducible promoter in pCM252 in strain CML282,
allowing us to regulate expression of Bax following addition of doxycycline to the media.25 When grown on media containing 1 μg/ml doxycycline, all three strains express similar levels of
Bax (Figure 1b).
To evaluate the ability of Bax to inhibit growth in the absence of CL and PG, we assessed the viability of these strains following serial dilutions of cells onto plates containing increasing
concentrations of doxycycline (Figure 1c). The viability of the wild-type strain is dramatically compromised at concentrations of doxycycline higher than 0.1 μg/ml, as is each of the mutant
strains; the apparent increase in sensitivity of the Δcls1 and Δpgs1 strains may reflect growth defects generated by these mutations. However, the levels of Bax required to kill wild-type
and mutant strains are identical, suggesting that the sensitivity to Bax in vivo is not dependent on the presence of either CL or PG.
We also assessed the ability of an antiapoptotic protein such as Bcl-XL to abrogate the cell killing effects of a proapoptotic molecule like Bax in strains devoid of CL or PG. In wild-type
cells, coexpression of Bcl-XL is able to protect cells completely from the toxic effects of Bax expression.16, 18, 20, 25 Accordingly wild-type, Δcrd1 and Δpgs1 strains containing Bax
expression plasmids were cotransformed with plasmids that mediate the expression of N-terminally, HA-tagged Bcl-XL in response to the addition of doxycycline to the media; similar levels of
Bcl-XL are expressed in response to doxycycline addition in each strain (Figure 1b). Strain viability was again tested by the serial dilution of each strain onto the plates with varying
concentrations of doxycycline (Figure 1d). The viability of wild-type, Δcls1 and Δpgs1 strains were completely restored following coexpression of Bcl-XL at all concentration of doxycycline,
suggesting that the antiapoptotic Bcl-2 proteins do not require CL or PG for their action.
Using similar systems, our earlier studies have defined the quantitative relations between antagonist actions of Bax and Bcl-XL. These studies led to a model in which the action of Bax and
Bcl-XL on the mitochondrial membranes involves the interaction of Bax and Bcl-XL with the hypothetical target in the outer mitochondrial membrane.25 In this model, Bax and Bcl-XL compete for
a common target; interaction of Bax with this target leads to outer membrane permeabilization and cell death, while Bcl-XL, which interacts with the target preferentially, prevents the
target from the interaction with Bax. Clearly, in this model the availability of the target would determine the sensitivity of the cell to both Bax-mediated killing and Bcl-XL-mediated
rescue. The fact that absence of CL or PG does not affect yeast cell sensitivity to the action of either Bax and Bcl-XL indicates that the amount of target, the ability of target to interact
with Bax and Bcl-XL and preference of the target for Bcl-XL remain unaffected by the absence of either CL or PG.
While the experiments described in this report demonstrate that the action of proapoptotic Bax and antiapoptotic Bcl-XL do not require CL or PG, the possibility that CL participates in the
mitochondrial response to apoptotic signals at a different point in the cascade remains open. It has been suggested that CL may participate in the release of cyt. c by retaining a fraction
of cyt. c bound to the outer surface of the inner mitochondrial membrane.10, 26 In addition, several reports suggest that CL, or CL metabolites, can partition to other intracellular
membranes during apoptosis.27 However, rather than active participation of CL, it may be that a decrease in CL levels due to downregulation of de novo synthesis of CL as well as increased
turnover of existing CL during apoptosis, as it has been demonstrated in cardiomyocyte model,28 or the participation of CL breakdown products play a role during PCD. The precise mechanisms
by which CL may participate in the apoptotic response by such mechanisms remain to be firmly established and are not addressed by the studies reported here.
We thank Dr. Peter Griač for helpful discussions. This work was supported by grants from the NIH to MF (GM035759) and WD (GM056389).
Vollum Institute, Oregon Health & Sciences University, 3181 SW Sam Jackson Park Road, Portland, OR, 97239, USA
Department of Biochemistry & Molecular Biology, University of Texas-Houston, Medical School, 6431 Fannin, Suite 6.200, Houston, TX, 77030, USA
Anyone you share the following link with will be able to read this content: