Transmembrane crosstalk between the extracellular matrix and the cytoskeleton
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Cell adhesions represent the interaction interfaces between cells and the extracellular matrix. Their study provides exciting insights into the interplay between physical forces and
molecular signalling in cell regulation.
Cells use transmembrane actin–integrin adhesion complexes as mechanosensors to probe the rigidity of the extracellular environment, mediate adhesion, trigger signalling, and remodel the
extracellular matrix (ECM).
Local physical forces induce transitions in the types and functions of these cell–matrix adhesions: Focal complexes transform into focal adhesions, which serve as the source of fibrillar
adhesions.
Integrin translocation appears to stretch fibronectin molecules, exposing cryptic sites that mediate matrix assembly into extracellular fibrils.
A key mechanism in these transitions appears to be conformational changes induced by force or a local reorganization of scaffold or signalling molecules to promote multimolecular assembly.
Both intracellular molecular-complex formation at adhesion sites and ECM assembly are regulated by Rho-family GTPases.
The identification of the full repertoire of adhesion-associated molecules.
Characterization of the molecular and cellular nature of the mechanosensors involved in cell–matrix adhesion.
Characterization of the regulation and functional integration of the various forms of adhesions, which change depending on the state of differentiation, tissue location, and application of
local forces.
Exploring the structure and function of cell–matrix adhesions in three-dimensional microenvironments in vivo and explaining the roles of complex carbohydrates in cell–matrix interactions.
Integrin-mediated cell adhesions provide dynamic, bidirectional links between the extracellular matrix and the cytoskeleton. Besides having central roles in cell migration and morphogenesis,
focal adhesions and related structures convey information across the cell membrane, to regulate extracellular-matrix assembly, cell proliferation, differentiation, and death. This review
describes integrin functions, mechanosensors, molecular switches and signal-transduction pathways activated and integrated by adhesion, with a unifying theme being the importance of local
physical forces.
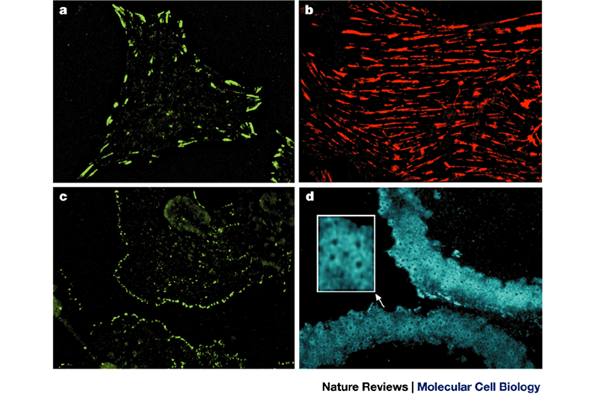
Figure 1a and b were kindly provided by E. Zamir, figure 1c by I. Grosheva and Figure 1d by J. Blair. B.G. is the incumbent of the E. Neter Chair in Cell and Tumor Biology, A.B. holds the J.
Moss Chair of Biomedical Research.
Department of Molecular Cell Biology, The Weizmann Institute of Science, Rehovot, 76100, Israel
Craniofacial Developmental Biology and Regeneration Branch, National Institute of Craniofacial and Dental Research, National Institutes of Health, Bethesda, 20892, MD, USA
A group of heterodimeric transmembrane adhesion receptors for extracellular-matrix proteins such as fibronectin and vitronectin.
A dense, sheet-like, laminated extracellular matrix that separates epithelia, muscle, or other tissues from connective tissue.
A thin, flat extension at the cell periphery, which is filled with a branching meshwork of actin filaments.
A family of monomeric G proteins — comprising Rho, Rac and Cdc42 — that are homologous to Ras. These are important molecular switches, which control cytoskeletal assembly and contraction.
A specialized cell that is involved in active bone resorption.
Subunits of fibronectin are comprised of repeating structural modules of three types (I, II, III). Each module is encoded by one or two exons with introns that precisely separate repeats.
There are 12 type I modules, each around 45 amino-acids long and clustered into three groups; two type 2 modules, each 60 amino acids-long; and 15–17 type III repeats, each about 90
amino-acids long (see Fig. 6).
(1-(5-iodonaphthalene-1-sulphonyl)-1-H-hexahydro-1, 4-diazepine) A kinase inhibitor thought to be relatively specific for myosin light-chain kinase.
(1-(5-isoquinolinylsulphonyl)-2-methylpiperazine) A broad-spectrum serine–threonine kinase inhibitor that blocks myosin light-chain kinase, Rho kinase and certain other kinases.
Microscope-based device that traps micron-sized particles in a focused laser beam. Can be used to move or to stop such particles.
The leading region of the advancing lamellipodium in a motile cell.
An approach for visualizing cellular contractility that is based on wrinkling of a thin and flexible silicone rubber film on which the cell is cultured.
A microscopic device in which cells are attached to a surface that consists of arrays of cantilevers. Local forces that are applied to this surface induce tilting of these cantilevers, which
can be measured.
Polymeric elastic gels that either contain impregnated beads or are surface micro-patterned are used as substrates for cultured cells. Local forces that are applied to these substrates can
be measured, based on the distortion of these patterns.
Tissues that form the architectural framework of the vertebrate body. In these tissues, the extracellular matrix is plentiful and cells are sparsely distributed within it.
A contractile, myofibroblast-containing tissue formed in wounds.
A condition in which contraction of muscle, non-muscle cells or the actomyosin network is opposed by an equal load that prevents net shortening, even though tension increases.
A macrolide that is derived from the Red Sea sponge Latrunculia magnifica, which binds and sequesters actin molecules, and thereby prevents the assembly of actin filaments.
A special state in polymer dynamics, when monomer addition at one end occurs at the same rate as monomer dissociation at the other end, which keeps the polymer length unchanged.
The fast-polymerizing end of actin filaments (defined by the arrowhead-shaped decoration of actin filaments with myosin fragments).
A computer simulation method for studying force-induced reactions in biopolymers.
The primary adhesive motif in many extracellular matrix molecules, which contains the amino-acid triplet, Arg–Gly–Asp.
An enzyme (such as factor XIIIa) that helps to crosslink fibronectin and other molecules through isopeptide linkages.
Anyone you share the following link with will be able to read this content: