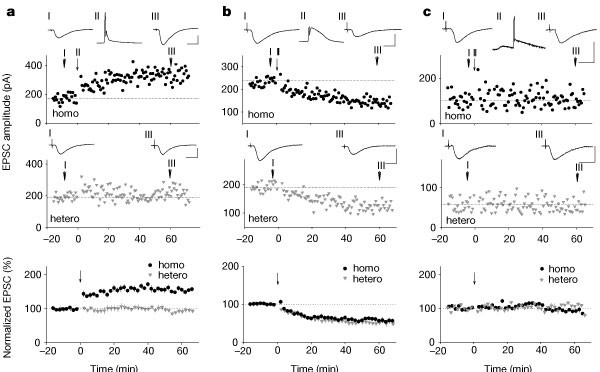
Calcium stores regulate the polarity and input specificity of synaptic modification
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Activity-induced synaptic modification is essential for the development and plasticity of the nervous system1,2,3. Repetitive correlated activation of pre- and postsynaptic neurons can
induce persistent enhancement or decrement of synaptic efficacy, commonly referred to as long-term potentiation or depression2,3(LTP or LTD). An important unresolved issue is whether and to
what extent LTP and LTD are restricted to the activated synapses4,5,6,7,8. Here we show that, in the CA1 region of the hippocampus, reduction of postsynaptic calcium influx by partial
blockade of NMDA (N-methyl-d-aspartate) receptors results in a conversion of LTP to LTD and a loss of input specificity normally associated with LTP, with LTD appearing at heterosynaptic
inputs. The induction of LTD at homo- and heterosynaptic sites requires functional ryanodine receptors and inositol triphosphate (InsP3) receptors, respectively. Functional blockade or
genetic deletion of type 1 InsP3 receptors led to a conversion of LTD to LTP and elimination of heterosynaptic LTD, whereas blocking ryanodine receptors eliminated only homosynaptic LTD.
Thus, postsynaptic Ca2+, deriving from Ca2+ influx and differential release of Ca2+ from internal stores through ryanodine and InsP3 receptors, regulates both the polarity and input
specificity of activity-induced synaptic modification.
We thank T. Michikawa for providing an antibody against InsP3R1; T. V. P. Bliss and R. C. Malenka for helpful discussions and suggestions; and N. Spitzer, J. R. Henley, D. Zacharias, A. F.
Schinder, S. Andersen and F. Engert for critical comments on the manuscript. This work was supported in part by a grant from USNIH.
Present address: Department of Biochemistry, New York University School of Medicine, 550 First Avenue, New York, New York, 10016, USA
Present address: Department of Molecular and Cell Biology, University of California, Berkeley, California, 94720, USA
Makoto Nishiyama and Kunio Kato: These authors contributed equally to this work.
Department of Biology, University of California at San Diego, La Jolla, 92093-0357, California, USA
Mikoshiba Calciosignal Net Project, Exploratory Research for Advanced Technology (ERATO), Japan Science and Technology Corporation (JST), Tokyo, 113-0021, Japan
Anyone you share the following link with will be able to read this content: