Intravenous busulfan for allogeneic hematopoietic stem cell transplantation in infants: clinical and pharmacokinetic results
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
High-dose busulfan is an important component of myeloablative regimens. Variable drug exposure may occur following oral administration. Therefore, the use of intravenous busulfan has been
advocated. Previous work has suggested a cumulative dosage of 16 mg/kg for haematopoietic transplantation in children less than 3 years of age, but only limited data are available in
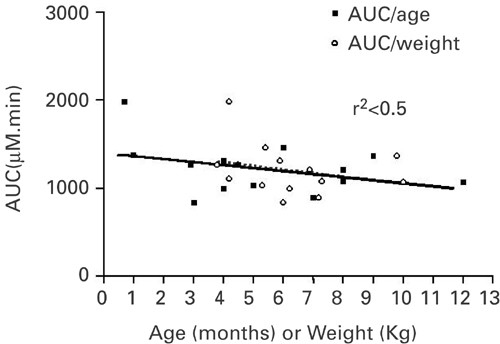
infants. Pharmacokinetics of intravanous busulfan administered at the suggested dosage were studied in 14 infants (median age 4.7 months). Busulfan plasma concentrations were measured by
either GC-MS or HPLC-UV. In seven patients, the dose was decreased to target an area- under- the- curve of 600–1300 μmol min. The median total dose given was 13.8 mg/kg. All patients
engrafted. Severe veno-occlusive disease occurred in one patient. Our study demonstrates that a cumulative dosage of 16 mg/kg is associated with higher exposure than expected in infants. We
suggest an initial dose of 0.8 mg/kg followed by pharmacokinetically guided dose adjustment.
Service d'Hématologie et Oncologie Pédiatrique, Hôpital Sainte Justine, Montréal, QC, Canada
J H Dalle, Y Theoret, M Duval, D Larocque, M F Vachon & M A Champagne
Methodist Children's Hospital of South Texas, San Antonio, TX, USA
Department of Pathology and Laboratory Medicine, University of Pennsylvania Medical Center, Philadelphia, PA, USA
Anyone you share the following link with will be able to read this content: