Blockade of the tumor necrosis factor-related apoptosis inducing ligand death receptor dr5 prevents β-amyloid neurotoxicity
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT We originally suggested that inhibition of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) death pathway could be taken into consideration as a potential therapeutic
strategy for Alzheimer's disease (AD). However, because the critical role of TRAIL in immune surveillance, the neutralization of TRAIL protein by an antibody to prevent its binding to
death receptors is definitely a risky approach. Here, we demonstrated that the blockade of the TRAIL death receptor DR5 with a specific antibody completely prevented amyloid _β_ peptide
(A_β_) neurotoxicity in both neuronal cell line and primary cortical neurons. DR5 was demonstrated to be a key factor in TRAIL death pathway. In fact, whereas TRAIL expression was enhanced
dose-dependently by concentrations of _β_ amyloid ranging from 10 nM to 1 μM, only the highest toxic dose of A_β_ (25 μM) induced the increased expression of DR5 and neuronal cell death. In
addition, the increased expression of DR5 receptor after _β_ amyloid treatment was sustained by p53 transcriptional activity, as demonstrated by the data showing that the p53 inhibitor
Pifithrin _α_ prevented both _β_ amyloid-induced DR5 induction and cell death. These data suggest a sequential activation of p53 and DR5 upon _β_ amyloid exposure. Further insight into the
key role of DR5 in AD was suggested by data showing a significant increase of DR5 receptor in cortical slices of AD brain. Thus, these findings may give intracellular TRAIL pathway a role in
AD pathophysiology, making DR5 receptor a possible candidate as a pharmacological target. SIMILAR CONTENT BEING VIEWED BY OTHERS THE ROLE OF MELATONIN IN AMYLOID BETA-INDUCED INFLAMMATION
MEDIATED BY INFLAMMASOME SIGNALING IN NEURONAL CELL LINES Article Open access 19 October 2023 MEK1/2 INHIBITION RESCUES NEURODEGENERATION BY TFEB-MEDIATED ACTIVATION OF AUTOPHAGIC LYSOSOMAL
FUNCTION IN A MODEL OF ALZHEIMER’S DISEASE Article Open access 10 August 2022 JNK INHIBITOR AND FERROPTOSIS MODULATOR AS POSSIBLE THERAPEUTIC MODALITIES IN ALZHEIMER DISEASE (AD) Article
Open access 07 October 2024 INTRODUCTION One of the more accredited candidates involved in etiopathology of Alzheimer's disease (AD) is thought to be the amyloid _β_ peptide (A_β_),
which is the major component of the extracellular deposition named senile plaques (Mori et al, 1992; LeVine, 2004; Heppner et al, 2004). Senile plaques, together with intracellular
depositions of neurofibrillary tangle, are the two anatomo-pathological hall markers that characterize this disease. A_β_ is a 39–43 amino-acid length peptide derived from the amyloidogenic
processing of the larger amyloid precursor protein (APP) (Haas et al, 1992; Seubert et al, 1992). The central role of A_β_ in AD is sustained by the discovery of APP mutation in some
familiar cases of the disease (Selkoe, 1996; Kowalska, 2003). This mutations have been demonstrated to be associated with an elevated production of A_β_ peptide 1–40 and 1–42, which have the
tendency to aggregate to form final unsoluble fibrils (Dewachter et al, 2000; Lewis et al, 2004; Helpern et al, 2004). The result coming from genetic studies and _in vitro_ and _in vivo_
experiments on animal models of AD gives A_β_ a neurotoxic effect (Cotman et al, 1992; Gray and Patel, 1995; Oster-Granite et al, 1996; Moechars et al, 1999; Ekinci et al, 2000; Peng et al,
2002; Tamagno et al, 2003; Rosales-Corral et al, 2004). It is well demonstrated that A_β_ causes different intracellular effects, involving different pathways that can proceed either
separately or sequentially. We have recently shown that one of the members of the TNF (tumor necrosis factor)-_α_ family, the TNF-related apoptosis inducing ligand (TRAIL), contributes
substantially to amyloid-induced neurotoxicity in human SH-SY5Y neuronal cell line (Cantarella et al, 2003). TRAIL is a novel peptide molecule belonging to the TNF family, whose main role is
to induce programmed cell death in tumor cell lines from various tissue origins (Pitti et al, 1996). TRAIL binds to five specific receptors of the TNF/NGF family (Pan et al, 1997a; Walczak
et al, 1997). However, only two of them, DR4 and DR5, have an intracellular death domain, which appears essential for triggering cell death processes (Pan et al, 1997a; Walczak et al, 1997).
Although the decoy receptors TRAIL-R3 and -R4 act as a dominant negative of TRAIL proapoptic effects (Pan et al, 1997b; Degli-Esposti et al, 1997; Sheridan et al, 1997), a fifth receptor,
named osteoprotegerin, appears to be involved in bone remodelling (Emery et al, 1998). The contribution of TRAIL to A_β_ neurotoxicity was demonstrated by data showing that
anti-TRAIL-neutralizing monoclonal antibody protects neuronal SH-SY5Y cells from A_β_ neurotoxicity (Cantarella et al, 2003). Furthermore, we recently showed TRAIL-positive cells in the
brain of AD patients. TRAIL staining was localized in the cerebral cortex of human brain slices, often in the proximity of Congo Red-positive amyloid plaques (Uberti et al, 2004). Thus,
these findings may give intracellular TRAIL pathway a role in AD pathophysiology, and indicate TRAIL peptide as a suitable target for pharmacological intervention. However, given the key
role of TRAIL in providing and maintaining immune surveillance, questions have been raised regarding the potential usefulness of an antibody anti-TRAIL in AD therapy. Thus, we addressed our
study toward another possible candidate belonging to the TRAIL pathway, the TRAIL DR5 receptor. Interestingly enough, TRAIL DR5 receptor is weakly expressed in human brain, and it is not
detectable in other human organs (Ichikawa et al, 2001). In contrast, many human cancer tissues reacted positively to anti-DR5 antibody. In the brain, DR5 receptor is substantially expressed
in neurons and oligodendrocytes, suggesting that the exposure to TRAIL could potentially induce apoptosis in these cells (Dorr et al, 2002). Consistent with this hypothesis, recombinant
TRAIL was found to induce apoptosis in human neuroblastoma cell line (Milani et al, 2003), rat cortical neurons and in human brain slices (Nitsch et al, 2000). In this study, we investigated
the involvement of TRAIL death receptor DR5 in A_β_-mediated apoptosis in both human neuronal cell line and mouse cortical neurons. Furthermore, we characterized the expression of the DR5
receptor in human brain derived from an AD case. MATERIALS AND METHODS CELL CULTURE Neuroblastoma cell line SH-SY5Y was routinely cultured in 1:1 Ham's F12:Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% (v/v) fetal calf serum, 2 mM glutamine, 50 μg/ml penicillin, and 100 μg/ml streptomycin and was kept at 37°C in a humidified 5% CO2/95% air.
For differentiation, cultures were seeded at approximately 105 cells/dish and retinoic acid was added to a final concentration of 10 μM. The medium was changed every day and cultures were
allowed to differentiate for 2 weeks. PRIMARY CULTURES OF MOUSE CORTICAL NEURONS Fifteen embryonic day mice were taken with Caesarean section from anesthetized pregnant dams. C57/BL6 mice
were purchased from Charles River, Italy. Cerebral cortices were isolated and dissociated by manual dispersion with a fire-polished Pasteur pipette. Cells were plated at a density of 1.5 ×
105 cells/cm2. Culture dishes were coated with 10 μg/ml poly-L-lysine. The cells were plated in Neurobasal medium (Invitrogen Corporation) supplemented with 2% B27 (Invitrogen Corporation),
0.5 mM L-glutamine, and 50 U/ml penicillin/streptomycin (Invitrogen Corporation). Three days after plating, 50% of the medium was changed with fresh medium and subsequently 50% of the medium
was changed twice a week, until 11 days _in vitro._ HUMAN TISSUES Brain tissues were removed for diagnostic purposes during autopsy from three AD patients and from one cognitive normal,
age-matched individual deceased owing to non-neurological disease. One AD case was a female died at the age of 78 years with a 5-year history of sporadic AD. The other two cases were
siblings (II-3, age of death 65 years, disease duration 7 years; II-5, age of death 56 years, disease duration 6 years) affected by a familiar form of AD caused by PS2 M239I mutation (Zatti
et al, 2004). The neuropathological diagnosis of AD was performed in accordance with CERAD recommendations (Mirra et al, 1991) by the local neuropathology unit at the Department of
Pathology. HANDLING OF _Β_-AMYLOID PEPTIDE A_β_1–42 and A_β_25–35 were purchased from Bachem Feinchemikalien AG (Bubendorf, Switzerland). Different lots of the peptides were dissolved in
sterile, doubly distilled water at a concentration of 2.5 mM and stored at −20°C. A_β_1–42 stock solutions were kept for 1 week in a 37°C incubator so as to allow aggregation and, therefore,
toxicity. A_β_25–35 was used immediately after suspension (Pike et al, 1993). All peptides were tested at graded concentrations (range: 10 nM–10 μM) for evaluation of cell toxicity.
EVALUATION OF CELL VIABILITY 5 × 103 cells/well were plated in 96-well plates and grown to 70–80% confluence in complete DMEM containing 10 μM retinoic acid. Differentiated cultures were
incubated for 48 h with 25 μM _β_AP25–35, or _β_AP1–42 in DMEM with 1% serum at 37°C. These agents were performed alone or in combination with Pifithrin _α_ (PIF) (200 nM), an inhibiting DR5
antibody (1 μg/ml; Alexis Biochemicals, San Diego, CA) or an isotype-matched control (IgG1 control) (1 μg/ml; Alexis Biochemicals, San Diego, CA). Cell viability was evaluated 48 h after
the addition of the cytotoxic agent to the media by measuring lactate dehydrogenase (LDH) activity in the culture-conditioned media. LDH activity was measured spectrophotometrically in the
culture media, following nicotinamide adenine dinucleotide (reduced form) oxidation at 340 nm. Total LDH activity was defined as the sum of intracellular and extracellular LDH activity.
Cytotoxicity was evaluated as percentage of total LDH activity. Each experiment was performed in triplicate and repeated at least twice. EVALUATION OF APOPTOSIS Terminal
deoxynucleotidyltransferase-mediated dUTP nick end labelling (TUNEL) was performed using the kit purchased by Roche Molecular Biochemicals according to the manufacturer's instructions.
The TUNEL-positive cells were counted in eight different fields for each well taken from experiments that were run in triplicate. For May-Grunwald–Giemsa's staining, cells were washed
in phosphate-buffered saline (PBS) solution, fixed with May-Grunwald's staining containing methanol for 3 min, then washed with a 200 mM NaH2PO4/Na2HPO4 buffer (pH 7.4) for additional 3
min. Cells were then stained (3 min) with Giemsa, and air-dried after three/four rapid washes in water. Cells were then analyzed under fluorescence microscope with × 40 objective and
photographed. IMMUNOFLUORESCENCE Cells were fixed in methanol for 5 min, raised in PBS and then incubated overnight with a polyclonal anti-TRAIL (Alexis) used at 1:500 dilution, a monoclonal
anti-_p53_ antibody (Ab 8, Neomarker) used at 1:300 dilution, or anti-DR5 (Alexis) used at 1:400 dilution. CyTM3-conjugated secondary antibodies were used. Slides were mounted with moviol
and were examined using a fluorescence microscope. WESTERN BLOT ANALYSIS Cells were harvested in 100 μl of lysis buffer containing 50 mM Tris, pH 7.6, 150 mM NaCl, 5 mM ethylene
diaminetetraacetic acid, 1 mM phenyl-methyl-sulfonyl-fluoride, 0.5 μg/μl leupeptin, 5 μg/μl aprotinin, 1 μg/ml pepstatin. The samples were sonicated and centrifuged at 15 000_g_ for 30 min
at 4°C. The resulting supernatants were isolated and the protein content was determined by a conventional method (BCA protein assay Kit, Pierce, Rockford, IL). Fifteen micrograms of total
protein were electrophoresed on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose paper (Schleicher and Schuell, Dassel, Germany). Filters were
incubated at room temperature overnight with anti-TRAIL (the same used for immunohistochemistry), anti-_p53_ (the same used for immunohistochemistry), anti-DR5 (C-20 Santa Cruz
Biotechnology), or anti-tubulin (Ab3, Neo Markers) antibody in 3% nonfat dried milk (Sigma). The secondary antibodies (Santa Cruz Biotechnology) and a chemiluminescence blotting substrate
kit (Boehringer, Mannheim, Germany) were used for immunodetection. Evaluation of immunoreactivity was performed on immunoblots by densitometric analysis using a KLB 2222–020 Ultra Scan XL
laser densitometer at a wavelength of 633 nm. STATISTICAL EVALUATION Results in cell viability and densitometric analysis of the immunoblots are given as mean±standard error mean values.
Statistical significance of differences was determined by mean values of the analysis of variance, following Student's test. Significance was accepted for a _p_-value of <0.05.
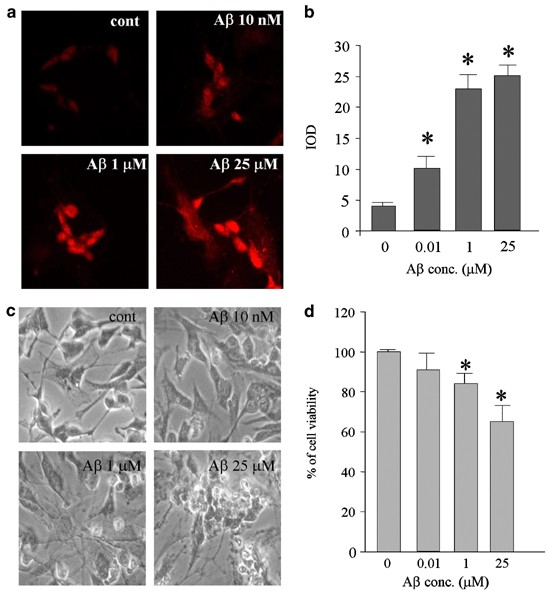
RESULTS TRAIL EXPRESSION IS NECESSARY BUT NONSUFFICIENT TO MEDIATE A_Β_ NEUROTOXICITY As previously described, high, neurotoxic doses of A_β_25–35 caused an increase expression of TRAIL that
was followed by apoptosis (Cantarella et al, 2003). Here, we investigate whether lower doses of A_β_ can modulate TRAIL expression on neuronal cell line SH-SY5Y. To that purpose, human
SH-SY5Y neuroblastoma cells were differentiated with retinoic acid for 1 week and then challenged with increasing concentrations of A_β_25–35, ranging from 1 nM to 25 μM, to evaluate cell
death and TRAIL protein expression. Interestingly, low concentration of A_β_25–35 (10 nM) was able to induce a significant increase of TRAIL expression and this effect proportionally
enhanced with the increase of A_β_ doses (Figure 1, panels a and b). Cell death was characterized by May-Grunwald–Giemsa's staining to visualize the late phase of apoptosis and by
measurement of extracellular LDH release. In line with previous observations (Mattson et al, 1992; Weiss et al, 1994), the neurotoxic effects induced by A_β_25–35 were found to be
concentration-dependent, being undetectable at 10 nM. Very few scattered apoptotic nuclei with a reduction of cell viability of about 10% were present at 1 μM concentration, whereas the
highest dose of 25 μM induced about 40% of cell death with evident sign of apoptosis (Figure 1, panels c and d). Altogether, these data raised the question of why, after exposure of the
cells to low concentrations of A_β_, the increased expression of TRAIL was not followed by apoptosis. Because TRAIL toxicity is mediated by its death receptors, we examined the expression of
the death receptor DR5 after different A_β_ concentrations. Figure 2, panel a shows a representative Western blot analysis performed on neuronal cells, carried out with anti-TRAIL and
anti-DR5 antibodies. In line with the immunofluorescence data reported above, TRAIL protein levels increased progressively with the increase of A_β_ doses. On the other hand, DR5 expression
was virtually absent in untreated cells and as well as in the cells exposed to 10 nM or 1 μM A_β_. The DR5 protein levels significantly increased only with toxic concentration (25 μM) of
A_β_ peptide (Figure 2, panel a). In this set of experiments, we also examined the expression of the tumor suppressor _p53_, because it is well recognized to be involved in A_β_
neurotoxicity and to be a transcriptional inducer of the DR5 gene (Wu et al, 1997). Like DR5, the _p53_ protein levels appeared significantly enhanced only at the toxic concentration of A_β_
(Figure 2, panel a). The quantitative analysis of immunoblot experiment performed on three different cell culture preparations confirmed the immunofluorescence data showing that A_β_
promotes a progressive concentration-dependent increase of TRAIL, but only at the proapoptotic concentration increases of DR5 and _p53_ expression (Figure 2, panel b). These data suggest a
critical role of the death receptor DR5 in TRAIL-mediated A_β_ neurotoxicity. DR5 EXPRESSION IS MEDIATED BY P53 IN HUMAN NEURONAL CELL LINE AND MOUSE CORTICAL NEURONS Neuronal cell lines
were preincubated with 200 nM PIF, a compound known to inhibit _p53_ activity and then exposed to 25 μM A_β_25–35 or to 10 μM A_β_1–42. At 8 h after the addition of A_β_ to the medium, the
cells were processed for immunofluorescence analysis carried out with anti-DR5 and anti-_p53_ antibodies. Both A_β_25–35 and A_β_1–42 induced a significant increase of DR5 immunoreactivity
that was prevented by the preincubation with PIF (Figure 3, panel a). Also, _p53_ immunostaining was found enhanced after A_β_25–35 and A_β_1–42 treatments, and the presence of PIF did not
alter A_β_-induced increase of _p53_ immunoreactivity (Figure 3, panel b). These results are consistent with those of Culmsee et al (2001), who suggest that the mechanism of action of PIF is
the inhibition of _p53_ nuclear translocation and prevention of its DNA-binding activity, rather than the downregulation of newly synthesized _p53_ or the inhibition of its activation by
relevant kinases. Similar results were obtained with cortical neurons exposed to 10 μM A_β_1–42. An intense cytoplasmic staining was observed in cortical neurons treated with A_β_1–42 at the
dose of 10 μM and immunostained with anti-DR5 antibody. Similarly to what was found in neuronal cell line, the inhibition of _p53_ activity by PIF prevented the induction of DR5 expression
induced by A_β_1–42 (Figure 3, panel c). Furthermore, many cortical neurons exposed to A_β_1–42 accumulated _p53_ protein in the nuclear compartment, whereas in some cells _p53_ remained
confined to the cytoplasm. Pretreatment with PIF did not modify _p53_ induction induced by A_β_1–42 (Figure 3, panel d). In support to immunofluorescence data, Western blot analysis
confirmed the direct correlation between _p53_ and DR5 receptor. In fact, an intense band representing DR5 protein levels was observed after A_β_1–42 exposure. DR5 protein levels returned to
basal levels after the combined treatment with PIF and A_β_1–42. Differently, PIF did not change the increased _p53_ protein levels induced by A_β_1–42 (Figure 3, panel e). The quantitative
analysis performed on three different cell culture preparations confirmed the data observed in the representative Western blot analysis. Both DR5 and _p53_ protein levels were increased
after treatment of the cells with A_β_1–42. The pretreatment with PIF prevented only A_β_1–42-induced increase of DR5 but not _p53_ expression (Figure 3, panel f). PIF AND ANTI-DR5 BLOCKING
PEPTIDE PREVENT A_Β_ NEUROTOXICITY IN NEURONAL CELL LINE AND CORTICAL NEURONS To give more insight into the intracellular cascades activated by A_β_, neuronal cell lines and cortical neurons
were treated with A_β_ peptides in the presence or absence of PIF or the anti-DR5 blocking peptide and then they were evaluated for cell viability. Treatment of neuronal cell line with
A_β_25–35 25 μM resulted in a reduction of cell viability by about 40%. A dose curve with fibrillar A_β_1–42 was also performed. The fibrillar peptide A_β_1–42 reduced cell viability in a
concentration-dependent manner (data not show). At the dose of 10 μM A_β_1–42 peptide cause a 65% of cell death. PIF partially but significantly rescued A_β_25–35 and A_β_1–42-induced cell
death (Figure 4, panel a). Interestingly, blockade of DR5 receptor with a specific anti-DR5 antibody completely prevented A_β_-induced cell death. These effects were specific as treatment of
the cells with a control isotype IgG1 did not affect A_β_-induced reduction of cell viability (Figure 4, panel b). Similar results were obtained with cortical neurons treated with A_β_1–42.
The neurotoxic effects of A_β_1–42 were partially abolished in cortical neurons pretreated with PIF (Figure 5, panel A). Cortical neurons were also treated with A_β_1–42 with or without
anti-DR5 antibody and 24 h later a TUNEL analysis was performed. As shown in Figure 5, panel B, few scattered TUNEL-positive cells were observed in untreated cells or in cells treated only
with anti-DR5 antibody (panel a and b). TUNEL-positive cells were found very abundant in the sample treated with A_β_1–42 alone (Figure 5, panel Bc), whereas the combined treatment of A_β_
with anti-DR5 antibody showed a pattern of cell viability similar to that found in untreated cells with very few apoptotic nuclei (Figure 5, panel Bd). These results were confirmed by LDH
release measurement, showing that anti-DR5 antibody prevented the cell loss induced by A_β_1–42 (Figure 5, panel C). DR5 RECEPTOR IS EXPRESSED IN AD BRAIN We previously found a significant
expression of TRAIL protein in the brain of AD patients, suggesting an involvement of TRAIL death pathway in the neurodegenerative process of AD (Uberti et al, 2004). Different studies have
also demonstrated the presence of TRAIL death receptor in human brain (Dorr et al, 2002; Nitsch et al, 2000). We had the opportunity to evaluate the expression of DR5 receptor in the brain
of three AD patients. The patients showed a history of AD, as designed according to the _Diagnostic and Statistical Manual of Mental Disorders_ (fourth edition) and the National Institute of
Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association clinical criteria. Postmortem examination confirmed the diagnosis of
AD because the presence of abundant Congo red-stained amyloid deposits and neurofibrillary tangles; hematoxylin–eosin staining also demonstrated marked cell loss (data not shown). Some
cortical brain slices were also analyzed for DR5 expression with an anti-DR5 antibody. The DR5-like immunoreactivity was then compared with the one found in the brain obtained from an
age-matched non-AD subject. As shown in a representative picture of brain slices derived from the sporadic AD patient (Figure 6), consistent and significant DR5 immunoreactivity was found in
the AD brain (panel b). At high magnification, the signal was found to be confined on cytoplasmic edge with a punctate staining (panel d). Similar results were obtained on brain slices
derived from the two familiar case of AD (data not shown). In brain slices from the control subjects, DR5 immunoreactivity was indeed found although at very low levels (panels a and c).
DISCUSSION It is well recognized that AD is a multifactorial disease in which many events occurring either separately or sequentially can contribute to the neurodegenerative process. In this
scenario, A_β_ seems to play a pivotal role. However, up to now, the mechanism behind A_β_-induced neurodegeneration is still controversial (Yankner et al, 1989; Geula et al, 1998). The
difficulty in understanding the A_β_ effects is sustained also by the fact that this toxic peptide can activate simultaneously different pathways that proceed separately or converge
together, and ultimately all lead to neuronal death. Identification of a preferential pathway could definitely be useful to design a new therapeutic drug to slow the progression of AD
pathology. Here, we show that the exposure of differentiated SH-SY5Y neuronal cells or primary cortical neurons to A_β_ peptide results in the induction of TRAIL, its death receptor DR5, and
_p53_. A dose–response study of the changes in TRAIL, _p53_, and DR5 expression demonstrated that A_β_ displays concentration-related effects characterized by the sequential involvement of
the three factors. In particular, low, nontoxic concentrations of A_β_ (nanomolar range) induced the expression of TRAIL without changing DR5 and _p53_ protein levels. High, neurotoxic
concentrations of A_β_ (micromolar range) caused an increased expression of all three proteins examined. Thus, in our experimental model, TRAIL was a necessary but not sufficient factor to
induce cell death upon A_β_ exposure. In this model, DR5 receptor appears to play a critical role as its expression was found to increase only after exposure to the highest, neurotoxic
concentration of A_β_. It has been shown that TRAIL binds to five specific receptors belonging to the TNF/NGF family (Pan et al, 1997a; Walczak et al, 1997). However, only two of them, DR4
and DR5, have an intracellular death domain, which appears essential for triggering cell death processes (Pan et al, 1997a; Walczak et al, 1997). In mice only DR5 receptor has been
identified (Wu et al, 1999). This receptor is a homolog of human DR5 and DR4 and binds TRAIL with an affinity similar to the one displayed by human DR4 and DR5 receptors. In this study, DR4
was not taken into consideration because we previously demonstrated that in differentiated SH-SY5Y cells, A_β_25–35 as well as TRAIL synthetic peptide did not alter the DR4 expression
(Cantarella et al, 2003). The most convincing result indicating the crucial role of DR5 in A_β_ neurotoxicity comes from the experiments showing that the DR5 blocking peptide completely
prevents A_β_-induced neurotoxicity in both neuronal cell line and cortical neurons. The earlier biochemical event following the engagement of DRs receptors by their ligand is the
recruitment of protein towards the intracellular death domain of the receptor to form a structure known as the death-inducing signalling complex (DISC) (Kischkel et al, 1995). DISC includes
the recruitment of FADD and the activation of pro-caspase 8 or pro-caspase 10. Caspase 8 activated the pro-caspase 3 and in turn they lead to apoptosis. Furthermore, caspase 8 can induce the
cleavage of bid, which translocates to the mitochondria where it activates bax and bak, providing a mechanism for crosstalk between the DRs and the apoptotic intrinsic pathway. The
correlation between DR5 and _p53_ comes from the identification of a subtractive hybridization screen for _p53_-target genes indicating DR5 gene as one of them. We found that the increased
expression of DR5 after A_β_ was prevented by PIF, a compound that inhibited _p53_ activity. _p53_ is a pleiotropic transcription factor that plays an important role in determining cell fate
under certain conditions (see review Almog and Rotter, 1997). _p53_ is constitutively present in many cell types including neurons and is upregulated and activated via phosphorylation
following various insults including excitotoxicity, ischemic injury, ionizing radiation, and oxidative stress (Crumrine et al, 1994; Sakhi et al, 1997; Uberti et al, 1998; Johnson et al,
1998; Inamura et al, 2000). A_β_-induced cytotoxicity is also demonstrated to involve _p53_ in primary cortical neurons (Copani et al, 2001) and different neuronal cell lines (Misiti et al,
2005; Matsumoto et al, 2006). In line with these latter studies, our data obtained with PIF demonstrated that A_β_ neurotoxicity is mediated by _p53_ in both neuronal cell line and cortical
neurons. In fact, inhibition of _p53_ activation prevented the A_β_-induced neurotoxicity. PIF is a synthetic inhibitor of _p53_ and was shown to protect against radiation, excitotoxicity,
and, as here demonstrated, against A_β_ toxicity (Culmsee et al, 2001). The postulated mechanism of PIF activity involved the inhibition of _p53_ nuclear translocation and prevention of its
binding to specific DNA sites and it is not related to downregulation of newly synthesized _p53_ or inhibition of _p53_ activation by relevant kinases (Culmsee et al, 2001). In line with
these evidences, we have shown that PIF did not alter A_β_-induced increase of _p53_ expression whereas it prevented the increased expression of DR5. _p53_ promotes cellular apoptosis
through transactivation of various pro-apoptotic genes that can act via an intrinsic pathway involving the mitochondria activation or an extrinsic pathway requiring activation of death
receptors. Although the extrinsic pathway and the intrinsic pathway for apoptosis are capable of operating independently, accumulated evidence suggests that a crosstalk between the two
pathways exists in cells (for a review see Green, 1998). The results obtained with anti-DR5 antibody indicated that the blocking of DR5 receptor completely prevented A_β_-induced
neurotoxicity in neuronal cultures. These data suggest that _p53_-mediated apoptosis may occur by a preferential activation of death receptors pathway. We cannot exclude the involvement of
intrinsic apoptotic pathway in A_β_ neurotoxicity. Indeed, our data suggest that the death receptor pathway, through the activation of caspase 8, can also activate the intrinsic pathway
through the cleavage of bid. The truncated Bid then translocates to mitochondria and triggers cytochrome _c_ release (Li et al, 1998; Luo et al, 1998). It has been proposed that Bid
regulates cytochrome _c_ release by inducing Bax translocation into mitochondria. In this regard, cells lacking Bax were resistant to TRAIL-induced apoptosis (Deng and Wu, 2000). Finally,
this study demonstrated a significant expression of DR5 receptor in AD brain. The expression of apoptosis-mediating TRAIL receptors indicates that brain cells, including neurons, are
potentially susceptible to TRAIL. Although it is unknown whether or not other TRAIL receptors are modulated in AD pathology, the upregulation of TRAIL death receptor DR5 together with the
presence of TRAIL in AD brain (Uberti et al, 2004) generate an appropriate environment to execute a cell death program. A possible role of TRAIL pathway in neurodegenerative diseases was
also suggested by Martin-Villalba et al (1999) who showed that, after middle cerebral artery occlusion in adult rats, TRAIL is expressed in the apoptotic areas of the postischemic brain.
Furthermore, TRAIL was found to be one of the major apoptotic factors inducing neuronal death in a murine model of HIV central nervous system infection (Miura et al, 2003; Ryan et al, 2004).
In conclusion, these data suggest a role of TRAIL death pathway in A_β_ neurotoxicity and propose DR5 receptor as a possible target for the development of neuroprotective drugs in those
neuropathologies in which TRAIL pathway plays a relevant role. REFERENCES * Almog N, Rotter V (1997). Involvement of p53 in cell differentiation and development. _Biochim Biophys Acta_ 1333:
F1–F27. CAS PubMed Google Scholar * Cantarella G, Uberti D, Carsana T, Lombardo G, Bernardini R, Memo M (2003). Neutralization of TRAIL death pathway protects human neuronal cell line
from beta-amyloid toxicity. _Cell Death Differ_ 10: 134–141. Article CAS Google Scholar * Copani A, Uberti D, Sortino MA, Bruno V, Nicoletti F, Memo M (2001). Activation of
cell-cycle-associated proteins in neuronal death: a mandatory or dispensable path? _Trends Neurosci_ 24: 25–31. Article CAS Google Scholar * Cotman CW, Pike CJ, Copani A (1992). Beta
amyloid neurotoxicity: a discussion of _in vitro_ findings. _Neurobiol Aging_ 13: 587–590. Article CAS Google Scholar * Crumrine RC, Thomas AL, Morgan PF (1994). Attenuation of p53
expression protects against focal ischemic damage in transgenic mice. _J Cereb Blood Flow Metab_ 14: 887–891. Article CAS Google Scholar * Culmsee C, Zhu X, Yu QS, Chan SL, Camandola S,
Guo Z _et al_ (2001). A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. _J Neurochem_ 77: 220–228. Article
CAS Google Scholar * Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG (1997). The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated
apoptosis, yet retains an incomplete death domain. _Immunity_ 7: 813–820. Article CAS Google Scholar * Deng Y, Wu X (2000). Peg3/Pw1 promotes p53 mediated apoptosis by inducing Bax
translocation from cytosol to mitochondria. _Proc Natl Acad Sci_ 97: 12050–12055. Article CAS Google Scholar * Dewachter I, Van Dorpe J, Smeijers L, Gilis M, Kuiperi C, Laenen I _et al_
(2000). Aging increased amyloid peptide and caused amyloid plaques in brain of old APP/V717I transgenic mice by a different mechanism than mutant presenilin1. _J Neurosci_ 20: 6452–6458.
Article CAS Google Scholar * Dorr J, Bechmann I, Waiczies S, Aktas O, Walczak H, Krammer PH _et al_ (2002). Lack of tumor necrosis factor-related apoptosis-inducing ligand but presence of
its receptors in the human brain. _J Neurosci_ 22: 1–5. Article Google Scholar * Ekinci FJ, Linsley M, Shea TB (2000). Beta amyloid-induced calcium influx induces apoptosis in culture by
oxidative stress rather than tau phosphorylation. _Mol Brain Res_ 76: 389–395. Article CAS Google Scholar * Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C _et al_ (1998).
Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. _J Biol Chem_ 273: 14363–14367. Article CAS Google Scholar * Geula C, Wu CK, Saroff D, Lorenzo A, Yuan M, Yankner BA (1998).
Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. _Nat Med_ 4: 827–831. Article CAS Google Scholar * Gray CW, Patel AJ (1995). Neurodegeneration mediated by
glutamate and beta-amyloid peptide: a comparison and possible interaction. _Brain Res_ 691: 169–179. Article CAS Google Scholar * Green DR (1998). Apoptotic pathway: the road to ruin.
_Cell_ 94: 695–698. Article CAS Google Scholar * Haas C, Scholossmacher MC, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL _et al_ (1992). Amyloid beta-peptide is produced by cultured
cells during normal metabolism. _Nature_ 359: 149–153. Article Google Scholar * Helpern JA, Lee SP, Falangola MF, Dyakin VV, Bogart A, Ardekani B _et al_ (2004). MRI assessment of
neuropathology in a transgenic mouse model of Alzheimer's disease. _Magn Reson Med_ 51: 794–798. Article Google Scholar * Heppner FL, Gandy S, McLaurin J (2004). Current concepts and
future prospects for Alzheimer disease vaccines. _Alzheimer Dis Assoc Disord_ 18: 38–43. Article CAS Google Scholar * Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T _et al_ (2001).
Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. _Nat Med_ 7: 954–960. Article CAS Google Scholar * Inamura N, Araki T, Enokido Y,
Nishio C, Aizawa S, Hatanaka H (2000). Role of p53 in DNA strand break-induced apoptosis in organotypic slice culture from the mouse cerebellum. _J Neurosci Res_ 60: 450–457. Article CAS
Google Scholar * Johnson MD, Xiang H, London S, Kinoshita Y, Knudson M, Mayberg M _et al_ (1998). Evidence for involvement of Bax and p53, but not caspases, in radiation-induced cell death
of cultured postnatal hippocampal neurons. _J Neurosci Res_ 54: 721–733. Article CAS Google Scholar * Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH _et al_ (1995).
Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. _EMBO J_ 14: 5579–5588. Article CAS Google Scholar *
Kowalska A (2003). Amyloid precursor protein gene mutations responsible for early-onset autosomal dominant Alzheimer's disease. _Folia Neuropathol_ 41: 35–40. CAS PubMed Google
Scholar * LeVine III H (2004). The Amyloid Hypothesis and the clearance and degradation of Alzheimer's beta-peptide. _J Alzheimers Dis_ 6: 303–314. Article CAS Google Scholar *
Lewis HD, Beher D, Smith D, Hewson L, Cookson N, Reynolds DS _et al_ (2004). Novel aspects of accumulation dynamics and A beta composition in transgenic models of AD. _Neurobiol Aging_ 21:
1175–1185. Article Google Scholar * Li P, Nijhawan D, Budihardjo I, Sirinvasula C, Wang X (1998). Cytochrome c and dATP-dependent formation of Apaf-1/caspase 9 complex initiates an
apoptotic protease caspase. _Cell_ 91: 479–489. Article Google Scholar * Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998). BID, a Blc2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface death receptors. _Cell_ 94: 481–490. Article CAS Google Scholar * Martin-Villalba A, Herr I, Jeremias I, Hahne M,
Brandt R, Vogel J _et al_ (1999). CD95 (FAS-L/APO-1L) and Tumour Necrosis Factor-Related Apoptosis-Inducing Ligand mediate ischemia-induced apoptosis in neurons. _J Neurosci_ 15: 3809–3817.
Article Google Scholar * Matsumoto K, Akao Y, Yi H, Shamoto-Nagai M, Maruyama W, Naoi M (2006). Overexpression of amyloid precursor protein induces susceptibility to oxidative stress in
human neuroblastoma SH-SY5Y cells. _J Neural Transm_ 113: 125–135. Article CAS Google Scholar * Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE (1992). beta-Amyloid
peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. _J Neurosci_ 12: 376–389. Article CAS Google Scholar * Milani D, Zauli G, Rimondi
E, Celeghini C, Marmiroli S, Narducci P _et al_ (2003). Tumour necrosis factor-related apoptosis-inducing ligand sequentially activates pro-survival and pro-apoptotic pathways in SK-N-MC
neuronal cells. _J Neurochem_ 86: 126–135. Article CAS Google Scholar * Mirra SS, Heyman A, McKell D, Sumi SM, Crain BJ, Brownlee LM _et al_ (1991). The Consortium to Establish a Registry
for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathological assessment of Alzheimer's disease. _Neurology_ 41: 479–486. Article CAS Google Scholar *
Misiti F, Sampaolese B, Pezzotti M, Marini S, Coletta M, Ceccarelli L _et al_ (2005). Abeta(31–35) peptide induce apoptosis in PC 12 cells: contrast with Abeta(25–35) peptide and examination
of underlying mechanisms. _Neurochem Int_ 46: 575–583. Article CAS Google Scholar * Miura Y, Misawa N, Kawano Y, Okada H, Inagaki Y, Yamamoto N _et al_ (2003). Tumour necrosis
factor-related apoptosis-inducing ligand induces neuronal death in a murine model of HIV central nervous system infection. _Proc Natl Acad Sci USA_ 100: 2777–2782. Article CAS Google
Scholar * Moechars D, Lorent K, Van Leuven F (1999). Premature death in transgenic mice that overexpress a mutant amyloid precursor protein is preceded by severe neurodegeneration and
apoptosis. _Neuroscience_ 91: 819–830. Article CAS Google Scholar * Mori H, Takio K, Ogawara M, Selkoe DJ (1992). Mass spectometry of purified amyloid beta protein in Alzheimer's
disease. _J Biol Chem_ 267: 17082–17086. CAS PubMed Google Scholar * Nitsch R, Bechmann I, Deisz RA, Haas D, Lehmann TN, Wendling U _et al_ (2000). Human brain-cell death induced by
tumour-necrosis-factor-related apoptosis-inducing ligand (TRAIL). _Lancet_ 356: 827–828. Article CAS Google Scholar * Oster-Granite ML, McPhie DL, Greenan J, Neve RL (1996). Age-dependent
neuronal and synaptic degeneration in mice transgenic for the C terminus of the amyloid precursor protein. _J Neurosci_ 16: 6732–6741. Article CAS Google Scholar * Pan G, Ni J, Wei Y, Yu
G, Gentz R, Dixit VM (1997b). An antagonist decoy receptor and a death domain-containing receptor for TRAIL. _Science_ 277: 815–818. Article CAS Google Scholar * Pan G, O'Rourke K,
Chinnaiyan AM, Gentz R, Ebner R, Ni J _et al_ (1997a). The receptor for the cytotoxic ligand TRAIL. _Science_ 276: 111–113. Article CAS Google Scholar * Peng QL, Buz'Zard AR, Lau BHS
(2002). Pycnogenol protects neurons from amyloid beta peptide-induced apoptosis. _Mol Brain Res_ 104: 55–65. Article CAS Google Scholar * Pike CJ, Burdik D, Walencewicz AJ, Gable CG,
Cotman CW (1993). Neurodegeneration induced by _β_-amyloid peptides _in vitro_: the role of peptide assembly state. _J Neurosci_ 13: 1676–1687. Article CAS Google Scholar * Pitti RM,
Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A (1996). Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. _J Biol Chem_ 271:
12687–12690. Article CAS Google Scholar * Rosales-Corral S, Tan DX, Reiter RJ, Valdivia-Velazquez M, Acosta-Martinez JP, Ortiz GG (2004). Kinetics of the neuroinflammation-oxidative
stress correlation in rat brain following the injection of fibrillar amyloid beta onto the hippocampus _in vivo_. _J Neuroimmunol_ 150: 20–28. Article CAS Google Scholar * Ryan LA, Peng
H, Erichsen DA, Huang Y, Persidsky Y, Zhou Y _et al_ (2004). TNF-related apoptosis-inducing ligand mediates human neuronal apoptosis: link to HIV-1-associated dementia. _J Neuroimmunol_ 148:
127–139. Article CAS Google Scholar * Sakhi S, Bruce A, Sun N, Tocco G, Baudry M, Schreiber SS (1997). Induction of tumor suppressor p53 and DNA fragmentation in organotypic hippocampal
cultures following excitotoxin treatment. _Exp Neurol_ 145: 81–88. Article CAS Google Scholar * Selkoe DJ (1996). Amyloid beta protein and the genetics of Alzheimer's disease. _J
Biol Chem_ 271: 18295–18298. Article CAS Google Scholar * Seubert PC, Vigo-Pelfrey C, Esch F, Lee M, Dovery H, Davis D _et al_ (1992). Isolation and quantification of soluble
Alzheimer's beta-peptide from biological fluids. _Nature_ 359: 325–327. Article CAS Google Scholar * Sheridan JP, Marsters S, Pitti RM, Gurney A, Skubatch M, Baldwin D _et al_
(1997). Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. _Science_ 277: 818–821. Article CAS Google Scholar * Tamagno E, Parola M, Gulglielmotto M, Santoro
G, Bardini P, Marra L _et al_ (2003). Multiple signaling events in amyloid beta induced oxidative stress-dependent neuronal apoptosis. _Free Radical Biol Med_ 35: 45–58. Article CAS
Google Scholar * Uberti D, Belloni M, Grilli M, Spano P, Memo M (1998). Induction of tumour-suppressor phosphoprotein p53 in the apoptosis of cultured rat cerebellar neurones triggered by
excitatory amino acids. _Eur J Neurosci_ 10: 246–254. Article CAS Google Scholar * Uberti D, Cantarella G, Facchetti F, Cafici A, Grasso G, Bernardini R _et al_ (2004). TRAIL is expressed
in the brain cells of Alzheimer's disease patients. _Neuroreport_ 15: 579–581. Article CAS Google Scholar * Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N
_et al_ (1997). TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. _EMBO J_ 16: 5386–5397. Article CAS Google Scholar * Weiss JH, Pike CJ, Cotman CW (1994). Ca2+ channel blockers
attenuate beta-amyloid peptide toxicity to cortical neurons in culture. _J Neurochem_ 62: 372–375. Article CAS Google Scholar * Wu GS, Burns TF, McDonald III ER, Jiang W, Meng R, Krantz
ID _et al_ (1997). KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. _Nat Genet_ 17: 141–143. Article CAS Google Scholar * Wu GS, Burns TF, Zhan Y, Alnemri ES,
El-Deiry S (1999). Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumour necrosis factor-related apoptosis –inducing ligand (TRAIL) death receptor.
_Cancer Res_ 59: 2770–2775. CAS PubMed Google Scholar * Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL (1989). Neurotoxicity of a fragment of the amyloid
precursor associated with Alzheimer's disease. _Science_ 245: 417–420. Article CAS Google Scholar * Zatti G, Gidoni R, Barbiero L, Binetti G, Pozzan T, Fasolato C _et al_ (2004). The
presinilin 2 M239I mutation associated with familial Alzheimer's disease reduces Ca2+ release from intracellular stores. _Neurobiol Dis_ 15: 269–278. Article CAS Google Scholar
Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biomedical Sciences and Biotechnologies, University of Brescia Medical School, Brescia, Italy Daniela Uberti,
Giulia Ferrari-Toninelli, Sara Anna Bonini, Ilenia Sarnico, Marina Benarese, Marina Pizzi, PierFranco Spano & Maurizio Memo * NeuroBioGen Lab-Memory Clinic, IRCCS ‘Centro San Giovanni di
Dio-FBF’, Brescia, Italy Luisa Benussi, Roberta Ghidoni & Giuliano Binetti * Department of Pathology, University of Brescia Medical School, Brescia, Italy Fabio Facchetti Authors *
Daniela Uberti View author publications You can also search for this author inPubMed Google Scholar * Giulia Ferrari-Toninelli View author publications You can also search for this author
inPubMed Google Scholar * Sara Anna Bonini View author publications You can also search for this author inPubMed Google Scholar * Ilenia Sarnico View author publications You can also search
for this author inPubMed Google Scholar * Marina Benarese View author publications You can also search for this author inPubMed Google Scholar * Marina Pizzi View author publications You can
also search for this author inPubMed Google Scholar * Luisa Benussi View author publications You can also search for this author inPubMed Google Scholar * Roberta Ghidoni View author
publications You can also search for this author inPubMed Google Scholar * Giuliano Binetti View author publications You can also search for this author inPubMed Google Scholar * PierFranco
Spano View author publications You can also search for this author inPubMed Google Scholar * Fabio Facchetti View author publications You can also search for this author inPubMed Google
Scholar * Maurizio Memo View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Daniela Uberti. RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Uberti, D., Ferrari-Toninelli, G., Bonini, S. _et al._ Blockade of the Tumor Necrosis Factor-Related Apoptosis Inducing Ligand
Death Receptor DR5 Prevents _β_-Amyloid Neurotoxicity. _Neuropsychopharmacol_ 32, 872–880 (2007). https://doi.org/10.1038/sj.npp.1301185 Download citation * Received: 21 March 2006 *
Revised: 13 June 2006 * Accepted: 27 June 2006 * Published: 16 August 2006 * Issue Date: 01 April 2007 * DOI: https://doi.org/10.1038/sj.npp.1301185 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * Alzheimer's disease * TRAIL * apoptosis * p53 * DR5 receptor * _β_ amyloid