Impact of catechol-o-methyltransferase on prefrontal brain functioning in schizophrenia spectrum disorders
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The enzyme catechol-_O_-methyltransferase (COMT) has attracted increasing interest regarding a genetic disposition towards schizophrenias and as a modulator of prefrontal brain
function. A common SNP in the _COMT_ gene causes a Val to Met transition at AA158/AA108 (Val158Met), resulting in reduced COMT activity in Met allele carriers. An impact of _COMT_ genotype
on cognition has been well established; however, the exact nature of this influence has yet to be elucidated. The aim of this study was to determine whether _COMT_ genotype affects an
electrophysiological marker of prefrontal activation and neuropsychological frontal lobe measures in schizophrenia. To this end, 56 acutely psychotic in-patients with schizophrenia spectrum
disorders were investigated. Patients with the _COMT_ 1947AA (Met/Met) genotype (_n_=13) were compared to a carefully matched sample of patients with a G1947A (Val/Met) genotype (_n_=15);
matching criteria included patients' age, handedness, gender distribution, diagnosis, and medication status. A small group of six homozygous Val allele carriers was additionally
included to allow an assessment of possible gene-dosage effects. P300 amplitudes and latencies, as well as an electrophysiological marker of prefrontal brain function
(NoGo-Anteriorization/NGA) and neuropsychological measures (Stroop Test, Verbal Fluency, Trail Making Test) were regarded. Homozygous Met allele carriers had significantly increased NGA
values and fronto-central Nogo amplitudes compared to patients with at least one Val allele. They also tended to perform better in the Stroop task, as compared to the matched group of
Val/Met patients. These results indicate that _COMT_ genotype exerts a strong impact on prefrontal functioning and executive control in schizophrenia spectrum disorders. SIMILAR CONTENT
BEING VIEWED BY OTHERS INFLUENCE OF CYTOCHROME P450 2D6 POLYMORPHISM ON HIPPOCAMPAL WHITE MATTER AND TREATMENT RESPONSE IN SCHIZOPHRENIA Article Open access 29 January 2021 PROCESSING SPEED
MEDIATES THE RELATIONSHIP BETWEEN _DDR1_ AND PSYCHOSOCIAL FUNCTIONING IN EUTHYMIC PATIENTS WITH BIPOLAR DISORDER PRESENTING PSYCHOTIC SYMPTOMS Article 19 February 2024
CATECHOL-_O_-METHYLTRANSFERASE RS4680 AND RS4818 HAPLOTYPE ASSOCIATION WITH TREATMENT RESPONSE TO OLANZAPINE IN PATIENTS WITH SCHIZOPHRENIA Article Open access 22 June 2020 INTRODUCTION
Schizophrenia is an etiologically heterogeneous group of disorders that involve serious alteration of the patients' cognitive, emotional, and social functioning. One of their cardinal
characteristics is a hypofunctionality of the frontal cortex (‘hypofrontality concept’; Ingvar and Franzen, 1974), which has been demonstrated in various studies and with different
methodological approaches (eg Andreasen et al, 1992; Fallgatter and Mueller, 2001). One of the affected areas is the anterior cingulate cortex (ACC), which has been shown to be deficient at
rest (Tamminga et al, 1992) as well as during activation with neuropsychological tasks (Andreasen et al, 1992; Carter et al, 1997). The ACC is critically involved in executive functions such
as the monitoring and regulation of ongoing actions, which usually comprise initiation of appropriate and inhibition of inappropriate actions. Tasks that typically involve both these
processes are Go–Nogo paradigms that demand the preparation and execution of responses to predefined target stimuli (Go) as well as the inhibition of prepared motor responses (Nogo). Over
the past years, an electrophysiological parameter has been developed and validated that supposedly reflects activation within prefrontal brain areas, particularly involving the ACC
(NoGo-anteriorization, NGA; Fallgatter et al, 1997). The NGA quantifies the anteriorization of the positive brain electrical field during the inhibition of prepared (motor) responses that is
usually observed during Go–Nogo tasks such as the Continuous Performance Test (CPT). Within the event-related potential (ERP), a marked positive component (P300) can be observed about 300
ms after presentation of a stimulus, for both Go and Nogo conditions of such a task. However, the topography of the P300 is usually located significantly more anterior during Nogo (response
inhibition) as compared to Go (response execution) trials (eg Bokura et al, 2001). This anteriorization of the brain electrical field can now be quantified by the NGA, which represents the
geometrical distance (or the arithmetical difference) between the center of gravity (‘centroid’) of the Go and Nogo P300 field distribution. In healthy subjects, the NGA was shown to have a
very high interindividual stability, excellent short- and long-term test–retest reliability, and it appears to be independent of the subjects' age and gender (reviewed by Fallgatter,
2001). In accordance with the results obtained by brain imaging studies (de Zubicaray et al, 2000; Rubia et al, 2001; Ford et al, 2004; Matthews et al, 2004), electrophysiological source
localizations (LORETA procedure; Pascual-Marqui et al, 1994) have revealed a close relationship between the NGA and a Nogo hyperactivity within prefrontal brain areas, particularly the ACC,
in healthy subjects (Strik et al, 1998; Fallgatter et al, 2002). Based on such findings, the NGA has been suggested to be an electrophysiological correlate of cognitive response control and
a neurophysiological marker of ACC function, even though other brain regions, particularly within the prefrontal cortex, are likely to be involved as well (eg Ford et al, 2004). In
accordance with the hypofrontality concept, schizophrenic patients were found to have a significantly diminished NGA (Fallgatter and Mueller, 2001) and a reduced activation of the ACC during
Nogo conditions (Fallgatter et al, 2003), findings that are again largely in line with electrophysiological (Kopp and Rist, 1999; Strandburg et al, 1999; Kiehl et al, 2000; Weisbrod et al,
2000; Mathalon et al, 2002) and neuroimaging data (Volz et al, 1999; Carter et al, 2001; Rubia et al, 2001; Laurens et al, 2003). The enzyme catechol-_O_-methyltransferase (COMT) has
gathered increasing interest in recent years with respect to the genetic disposition towards schizophrenia and as a modulator of prefrontal function in schizophrenic patients and healthy
controls. COMT degrades catecholamines and is thought to compete for dopamine removal from the synaptic cleft with the dopamine transporter. As the latter is abundantly expressed in the
striatum, COMT does not seem to play a major role here; however, in the prefrontal cortex, COMT is thought to be crucially involved in dopamine metabolism (Weinberger et al, 2001).
Schizophrenia has a substantial heritability of >80%, as estimated in a recent meta-analysis (Sullivan et al, 2003). Thus, huge efforts have been made to identify disease genes.
Chromosome 22q has been described as one of the confirmed regions for susceptibility loci (Badner and Gershon, 2002). Interestingly, the gene encoding COMT resides at 22q11, which makes it
an attractive candidate gene. A common SNP was found in the _COMT_ gene causing a Val to Met transition at amino-acid position 158 (or 108, respectively), which is commonly designated as
Val158Met. The Met allele codes for a ‘thermo-labile’ enzyme displaying lower enzymatic activity, resulting in increased synaptic dopamine and strengthened (prefrontal) dopaminergic tone.
Met/Met homozygotes display approximately 25% COMT activity compared to Val/Val homozygotes, with heterozygotes in-between. _COMT_ was therefore one of the prime candidate genes to identify
disease genes for psychosis, and Val158Met is currently one of the most frequently studied polymorphisms in schizophrenia. The results of association studies, however, are somewhat ambiguous
with both positive (eg Wonodi et al, 2003) and negative (Inada et al, 2003) findings. A recent haplotype analysis (Sanders et al, 2005) added further evidence for _COMT_ making a
contribution to the genetic risk of schizophrenia, as did a study utilizing a large number of Irish schizophrenia high-density families (Chen et al, 2004). Importantly, in both cases, the
over-transmitted haplotype included the Val allele. At a functional level, the impact of _COMT_ genotype on cognition has gathered increasing interest in recent years. Evidence for the
influence of _COMT_ on cognitive abilities came from _COMT_ knockout mice that were found to display improved performance in a memory task (Kneavel et al, 2000). Val158Met thereafter has
been repeatedly examined regarding its association with cognitive functioning, and in a seminal study Weinberger and associates showed that the Val allele causes reduced performance in the
Wisconsin Card Sorting Test (thought to mirror frontal executive functioning) in schizophrenic patients as well as healthy controls (Egan et al, 2001), a finding that could be replicated in
numerous subsequent studies. Regarding other neuropsychological functions, Val158Met was also found to influence processing speed and attention in chronic schizophrenic patients (Bilder et
al, 2002) and working memory in schizophrenics, their siblings, and controls (Goldberg et al, 2003). In a recent study (Nolan et al, 2004), schizophrenic Met allele carriers displayed better
cognitive _stability_, whereas subjects with the Val allele showed better cognitive _flexibility_. Thus, _COMT_ apparently plays a role in some cognitive domains, although its exact
contribution to functional skills has yet to be elucidated. One approach to further clarify the meaning of _COMT_ for cognitive functioning and underlying cerebral mechanisms is the
examination of neurophysiological parameters that reflect basic mechanisms of brain activation during the performance of cognitive operations and that are likely to be more directly linked
to underlying genomic variation than a highly variable behavioral phenotype. Compared to association studies with genetically complex behavioral traits, which frequently comprise several
hundreds of subjects, robust gene–brain activity correlations allow the investigation of substantially smaller sample sizes (Egan et al, 2001; Fallgatter et al, 2004). As the NGA has been
suggested to be an electrophysiological correlate of prefrontal functioning and is closely related to fundamental cognitive processes of executive control, it might be a promising research
parameter in attempts to further examine the impact of _COMT_ genotype on such processes. Considering the above-mentioned findings, we reasoned that _COMT_ genotype might influence this
electrophysiological marker in schizophrenia spectrum disorders. Therefore, the NGA was recorded in 34 carefully selected psychotic patients and correlated to Val158Met. PATIENTS AND METHODS
PATIENTS A total of 56 acutely psychotic psychiatric in-patients who were suffering from schizophrenia spectrum disorders were investigated. Exclusion criteria were age below 18 and above
60 years, comorbidity with other currently present axis-I disorders, a history of or an actually manifest disease of the CNS, or other severe somatic diseases. Thirteen patients with a
_COMT_ 1947AA genotype (homozygous Met allele carriers) had a sufficient number of at least 20 artifact-free ERP epochs, and could therefore be included in the present analysis (eight male,
10 right-handed, mean age 39.8±7.0 years). To ensure proper matching, 15 out of 22 patients with a G1947A genotype (Val/Met allele carriers) were selected to closely resemble the AA sample
in age, handedness, and gender distribution (seven male, 13 right-handed, 38.6±11.4 years), as well as diagnoses and medication status (antipsychotics and co-medication, see below). The
matching procedure was performed meticulously and before all data analyses. As a result, the selected group of Val/Met carriers did not differ significantly from the Met/Met group in any of
the above-mentioned parameters (_t_age=0.34, _p_=0.74; _t_cpz (daily chlorpromazine equivalents in mg (see below))=1.05, _p_=0.30; all _χ_2<0.8, _p_>0.4). A small group of six
homozygous Val allele carriers (three male, six right-handed, mean age 30.7±9.3 years) was additionally included to allow an assessment of gene-dosage effects, at least on a descriptive
level. As this last group was very small and thus could not be strictly matched to resemble the other two genotype groups, it was only included in additional non-parametric statistical
analyses (see below), which should be regarded as exploratory. According to the SKID-I-Interview, patients were diagnosed as disorganized (295.10; _n_=6), catatonic (295.20; _n_=4), paranoid
(295.30; _n_=11), and undifferentiated (295.90; _n_=3) types of schizophrenia, schizophreniform (295.40; _n_=8), or schizoaffective disorders (295.70; _n_=2). The mean duration of the
disease was 172±104 months (mean±SD), with an average of 6.9±7.5 admissions to psychiatric hospitals. Five patients had a positive family history of schizophrenia; another 10 patients had
first-degree relatives with non-psychotic or unknown psychiatric conditions. No significant current comorbidities were found in either patient sample except for one patient with
neuroleptic-induced adiposity and one with hypertonia; in the Val/Met group, two patients had life-time comorbidities (Bulimia nervosa; anxiety disorder), in the Met/Met group one patient
had a previous anorexia nervosa. Neuroleptic treatment consisted of 529±400 mg chlorpromazine equivalents per day; 13 patients were treated with typical antipsychotics, 15 patients received
atypical antipsychotics, and six patients no neuroleptic medication. As already mentioned above, Val/Met and Met/Met patients did not differ significantly regarding the diagnoses, nor the
amount or type of antipsychotic medication. Co-medications were regarded in the matching procedure as well, so that the mean daily doses did not differ significantly between the two
genotypes (_t_<1.2, _p_>0.25) for any of the potentially relevant substance groups (carbamazepin, biperiden, lithium, lorazepam, valproic acid, SSRI). Written informed consent was
obtained from all patients after the procedures had been fully explained. The study was approved by the Ethics Committee of the University of Wuerzburg, and the procedures involved were in
accordance with the Declaration of Helsinki. PSYCHOPATHOLOGICAL AND NEUROPSYCHOLOGICAL ASSESSMENT Each patient underwent an extensive psychometric examination, consisting of the
SKID-I-Interview, the Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962), the Positive and Negative Symptoms Scale (PANSS; Kay, 1991), and the Hamilton Depression Rating Scale
(HDRS; Hamilton, 1960). The neuropsychological assessment consisted of the Verbal Fluency Test (VFT), a Stroop Color Word Task, and the Trail Making Test (TMT). For the VFT, patients were
instructed to name as many nouns as possible beginning with a certain letter (‘letters version’) or belonging to a certain category of words (‘categories version’). The Stroop task consisted
of three parts, with two control conditions (‘word reading’, ‘color naming’) and one interference condition (color words were presented in a color that did not correspond to the word
meaning of the color word, and patients were instructed to name the ink color of the words). For the TMT, patients had to connect the numbers 1–15, randomly distributed on a sheet of paper,
in the correct order (Part A), or connect the numbers 1–8 and the letters A–G in the correct order while consecutively alternating between numbers and letter (Part B). ELECTROPHYSIOLOGICAL
INVESTIGATION The electrophysiological investigation took place in an electrically shielded, dimly lit room where the participants performed a CPT. Letters were presented sequentially in a
pseudo-randomized order and the patients had to press a response button whenever the letter ‘O’ (Primer) was directly followed by an ‘X’ (Go condition). The whole stimulus set consisted of
400 letters, with 114 primer stimuli, 57 Go and Nogo conditions (O followed by any other letter), and 172 distractors. Each letter was presented for 200 ms with an interstimulus interval of
1650 ms. The recording sessions took place between 0800 and 1200. The EEG was recorded from 21 scalp electrodes placed according to the International 10–20 system (Jasper, 1958) with three
additional electrodes to monitor eye movements. Linked mastoids were used as the recording reference; electrode impedances were below 5 k_Ω_. The recording system involved a 32-channel DC
amplifier (Brain Star System) and the Neuroscan data acquisition software calibrated with an external 100 μV/10 Hz signal. With an A/D rate of 256 Hz, the hardware filter was set to a
bandpass of 0.1–70 Hz. DATA ANALYSIS Data were analyzed offline with the program ‘Vision Analyzer’ (Brain Products, Munich, Germany). After re-referencing the data to an average reference,
they were segmented according to the conditions of the CPT (segments from −100 to 700 ms after stimulus presentation), and the Go and Nogo epochs were further analyzed. A computerized
artifact rejection excluded all segments with amplitudes exceeding ±50 μV; if at least 20 artifact-free EEG epochs were available for the Go and the Nogo condition, the remaining segments
were averaged to one Go and one Nogo ERP per patient. In the individual ERPs, the global field power (GFP; Lehmann and Skrandies, 1980) peaks were determined within a P300 time frame
(275–530 ms; based on a visual inspection of the grand average curves). The GFP represents the mean of all possible potential differences in a given scalp potential field and is used as a
measure of the amount of activity in this field. At the individual GFP peaks, the amplitude, latency, and anterior–posterior location of the positive centroid (the amplitude-weighted center
of gravity of the positive brain electrical field; Lehmann, 1987) were calculated. The centroid locations were quantified by a coordinate system defined by a two-dimensional delineation of
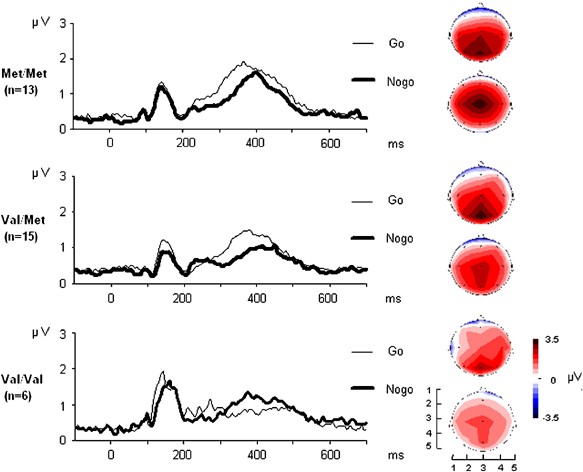
the electrode array (cf Figure 1). For the purpose of the present study, only the centroids in the anterior–posterior direction were of interest, the more anterior locations of the centroids
being represented by smaller numbers on this axis (eg ‘1’ represents electrode position Fpz, ‘5’ represents Oz). Finally, the NGA, defined as the distance between the individual Go and Nogo
centroid within the coordinate system (unit of the NGA=‘electrode positions’), was calculated. In addition to this topographical analysis, the P300 peaks were determined for the three
midline electrode positions (Fz, Cz, Pz) employing a semi-automatic peak picking procedure and using the same time frame mentioned above (275–530 ms post-stimulus); amplitudes and latencies
of these peaks were analyzed. GENOTYPING DNA was extracted from whole blood. Genotyping for _COMT_ G1947A SNP was accomplished using standard PCR procedures modified from a previously
published protocol (Egan et al, 2001); primers were 5′-GGG GCC TAC TGT GGC TAC TC-3′ (forward) and 5′-TTT TTC CAG GTC TGA CAA CG-3′ (reverse). Briefly, PCR reactions were performed in a
reaction volume of 25 μl, including approximately 50 ng of template genomic DNA, 10 pmol of each primer, 2.5 mM of each dNTP, 0.75 mM MgCl2, and 1 U of _Taq_ DNA polymerase. Annealing
temperature was 58°C (35 cycles). PCR products were digested with _Nla_III (3 h at 37°C; fragment sizes: wild-type G1947, 114 bp; 1947A variant, 96 and 13 bp) and subsequently visualized on
a 4% agarose gel. G1947 corresponds to the high-activity Val158 allele; 1947A codes for the low-activity Met variant. STATISTICAL ANALYSIS Only the data of Met/Met and Val/Met carriers were
subjected to parametric statistical analyses, as only these two groups were properly matched and included a sufficient number of patients per group. For the P300 centroids of these two
groups, a 2 × 2 analysis of variance (ANOVA) for repeated measurements was conducted, with the within-subject factor ‘condition’ (Go, Nogo) and the between-subject factor ‘group’ (Met/Met
_vs_ Val/Met). _Post hoc_ analyses were conducted by means of two-tailed _t_-tests for matched or independent samples. _T_-tests were also used to compare the single electrode (peak) ERP
data, the NGA, and the behavioral performance between the two groups. Equality of variances was tested by means of Levene's test, and corrections for inequality were performed whenever
necessary. As the number of commission errors was not normally distributed (Kolmogorov–Smirnov _Z_=1.54, _p_<0.05), between-group comparisons for this parameter were conducted by means of
Mann–Whitney _U_-tests. All the other electrophysiological and behavioral parameters were normally distributed (Kolmogorov–Smirnov _Z_<1.2, _p_>0.2) and were therefore subjected to
parametric testing procedures. Regarding the third genotype group (Val/Val; _n_=6), supplementary non-parametric test procedures were additionally conducted to investigate an effect of the
factor ‘genotype’ on neurophysiological and behavioral parameters across the three _COMT_ polymorphism groups (Kruskal–Wallis tests with Mann–Whitney _U_-tests for _post hoc_ comparisons).
Because of the small number of patients included in the third genotype group, however, this additional statistical analysis should be regarded as preliminary and the results should be
interpreted carefully. RESULTS PERFORMANCE MEASURES, PSYCHOMETRY, NEUROPSYCHOLOGY Reaction times, commission errors (button-press after non-target stimulus), and omission errors (no response
to Go stimulus) were used as performance measures for the CPT. Statistical testing revealed no significant differences between the two matched groups (Met/Met and Val/Met) for any of the
behavioral measures (reaction times: 633±200 _vs_ 606±126 ms, _t_26=0.44, NS; omission errors: 8.6±9.5 _vs_ 8.5±9.4 errors, _t_26=0.02, NS; commission errors: 2.3±3.3 _vs_ 1.1±1.4 errors,
_U_=84.5, NS) (Val/Val: RT=580±86 ms; 3.33±2.50 omission errors; 1.83±2.40 commission errors; Kruskal–Wallis _χ_2=0.73, 1.74, and 0.44, respectively; _p_>0.4). Regarding the different
psychometric scales, the two groups did not differ significantly either, even though patients with a Met/Met genotype exhibited slightly higher scores on most of them (Table 1). Regarding
the neuropsychological tests, Met/Met and Val/Met patients did not differ significantly in their VFT (letters: 21.7±9.8 _vs_ 24.2±12.1 words, _t_26=0.60, NS; categories: 29.1±10.4 _vs_
32.9±10.5 words, _t_25=0.97, NS) (Val/Val: 18.5±7.1 and 29.0±10.6 words, respectively; Kruskal–Wallis _χ_2<3.8, _p_>0.15) or TMT performance (A: 28.2±19.4 _vs_ 30.7±12.1 s, _t_26=0.42,
NS; B: 54.9±34.8 _vs_ 70.2±46.0 s, _t_26=0.98, NS) (Val/Val: 21.0±7.6 and 58.0±31.7 s in TMT A and B; Kruskal–Wallis _χ_2<3.8, _p_>0.15); however, for the interference condition of
the Stroop Test, the group of patients with a heterozygous genotype exhibited a statistical trend for prolonged times compared to the Met/Met group (116.8±35.0 _vs_ 146.4±54.7 s, _t_24=1.73,
_p_<0.1) (Val/Val: Stroop interference: 138.7±66.7 s; Kruskal–Wallis _χ_2<3.8, _p_>0.15). For the two control conditions of the Stroop task, on the other hand, a similar trend
could not be detected, the time needed to accomplish the tasks being very similar for all groups (data not shown). NGA AND ERP DATA Regarding the topographical ERP analysis, the ANOVA for
the positive centroids revealed a significant main effect of the factor ‘condition’ (F1, 26=9.18, _p_<0.01) and a significant interaction ‘condition × group’ (F1, 26=5.99, _p_<0.05).
Regarding the main effect, the centroid was located more anteriorly in CPT Nogo trials (3.2±0.1) as compared to Go conditions (3.5±0.1), which is a common finding (‘NGA’) even more markedly
visible in healthy subjects. Regarding the interaction, the Nogo-related anteriorization of the brain electrical field was much more pronounced in patients carrying Met/Met (Go _vs_ Nogo
centroid: 3.54±0.66 _vs_ 3.08±0.43, _t_12=3.75, _p_<0.01) than in the Val/Met group (3.41±0.62 _vs_ 3.36±0.69, _t_14=0.43, NS; Figure 1). This finding is confirmed by a significantly
reduced NGA in the group of Val/Met patients compared to the Met/Met group (Table 2). Homozygous Val allele carriers (_n_=6) showed an even further reduced mean NGA (−0.37±1.00), a
Kruskal–Wallis test confirming an effect of the factor ‘genotype’ on the NGA across the three groups (_χ_2=7.25, _p_<0.05) with a significantly larger NGA in Met/Met patients as compared
to Val allele carriers (Val/Met: _U_=48.0, _p_<0.05; Val/Val: _U_=15.0, _p_<0.05). Thus, the Val allele caused a reduced anteriorization of the brain electrical field during Nogo
conditions in a linear way (gene-dose effect). These topographical findings were largely reflected by the single electrode ERP data (Table 2). Regarding the two matched groups of patients,
Val/Met and Met/Met subjects did not differ significantly regarding any of the ERP measures elicited by Go trials. For the Nogo condition, however, Val/Met patients exhibited significantly
decreased P300 amplitudes at fronto-central electrode sites, and it is this failure to generate a robust fronto-central P300 during the CPT Nogo condition that underlies the reduced NGA we
observed in this group of patients. When taking into account the group of Val/Val patients as well, a Kruskal–Wallis test confirmed a statistical trend for a genotype effect on the Cz
amplitude only (_χ_2=5.01, _p_<0.1), Met/Met patients showing increased amplitudes as compared to Val allele carriers (Val/Met: _U_=54.5, _p_<0.05; Val/Val: _U_=19.0, _p_<0.1) All
three groups exhibited very similar P300 latencies, with no significant differences between the different genotypes (data not shown). To ensure that between-group differences were not caused
by differences in the number of ERP epochs included in the analyses, the number of artifact-free ERP epochs was determined for each group. With a mean of 43.2±11.2, 43.2±9.2, and 49.0±5.7
Go epochs, as well as 48.6±8.1, 47.6±7.6, and 51.0±3.0 Nogo segments included in the analysis of patients with a Met/Met, Val/Met, and Val/Val genotype, respectively, the three groups did
not differ significantly in either condition (Go: F2, 31=0.91; _p_=0.41; Nogo: F2, 31=0.47; _p_=0.63). DISCUSSION We conducted an electrophysiological and neuropsychological assessment of
patients with schizophrenia spectrum disorders stratified for COMT Val158Met polymorphism. In contrast to rather small effects on neuropsychological measures, the Val allele impacted heavily
on the NGA, an electrophysiological marker of prefrontal brain function. Val allele carriers had a virtually absent anteriorization of the brain electrical field during Nogo trials,
indicating reduced activation within prefrontal brain regions during conditions that impose increased demands on cognitive response control. This effect occurred in a gene-dosage-dependent
manner, with Met/Met patients showing the largest, Val/Met patients an intermediate, and Val/Val patients a very small, even negative mean NGA. This indicates that patients with a
particularly strong dopaminergic tone showed most consistently the expected functional activation pattern in a task involving prefrontal engagement. This is in accordance with previous
studies pointing towards a role of _COMT_ genotype in cognition, particularly with respect to prefrontal brain functions (see Introduction). This topographical effect was also partially
reflected in the single electrode ERP data, which showed a modulation by _COMT_ genotype in a very similar way, albeit not as strongly and consistently. In line with the present findings, we
showed in a preliminary report that two subjects suffering from 22q11 deletion syndrome and thus hemizygous for _COMT_ featured an absent NGA as well (Reif et al, 2004), further
underscoring the results of the present study. In the present study, only patients with schizophrenia spectrum disorders were investigated. Thus, no statement on the electrophysiological
impact of the COMT Val158Met polymorphism in healthy control subjects as compared to schizophrenic patients can be made on the basis of the present data. There are, however, numerous studies
reporting a detrimental effect of the Val allele on prefrontal functioning (neuropsychological and neuroimaging data) in both schizophrenic patients and healthy controls (for review see
Tunbridge et al, 2006; Craddock et al, 2006). As an underlying explanatory model, Weinberger and colleagues suggested an inverted U-shaped relation between cortical dopamine and prefrontal
cortex function, the precise effect of COMT activity depending on the basic dopaminergic tone of a given individual on this U-shaped curve. They furthermore suggest that in healthy controls
without pharmacological intervention, individuals with a Met/Met phenotype are located around the peak of the inverted U-shaped curve, with Val/Met and Val/Val carriers located slightly
further down along the curve's rising ‘left’ arm (Tunbridge et al, 2006). This would account for the replicated finding of a dose-dependent positive influence of the Met allele on
prefrontal (cognitive) functioning. Assuming that schizophrenic patients show hyperactive mesolimbic dopamine projections, but _hypoactive_ mesocortical dopamine projections to the
prefrontal cortex (eg Abi-Dargham and Moore, 2003), they should generally be located further down towards the beginning of the rising part of the inverted U-shaped curve. The principal
effect of _COMT_ genotype should therefore be similar in healthy controls as compared to the group of patients investigated here, although it is probably enhanced in schizophrenic patients
because of their abnormal dopaminergic state (steeper gradient of the curve towards its beginning). In summary, based on these considerations, healthy controls should be ‘superior’ to
schizophrenic patients regarding their prefrontal functioning irrespective of _COMT_ genotype; but _within_ the group of healthy controls, _COMT_ should exert a similar—albeit weaker—effect
as compared to patients with schizophrenia spectrum disorders. Two previous reports focused on electrophysiological measures as a function of _COMT_ genotype. Tsai et al (2003) examined
healthy female subjects and found that Met allele carriers had significantly reduced P300 latencies in a gene-dose-dependent manner. Gallinat et al (2003), on the other hand, investigated
schizophrenic patients and healthy controls by means of an auditory oddball paradigm without finding an effect of COMT genotype on P300 latencies. However, fronto-central P300 amplitudes
were lower in 158Met homozygous subjects, particularly in schizophrenia. This led the authors to the conclusion that in schizophrenics homozygous for the Met allele, less prefrontal cortical
‘noise’ occurred, which might be involved in the superior performance of Met allele carriers with regard to working memory and information processing. As the authors employed a paradigm in
which the frontal component of the P300 can be considered as a correlate of cortical noise, whereas other tasks (such as the CPT) involve frontal P300 components with strong ‘signal’
properties, the results reported by Gallinat _et al_ do not contradict the present data. In fact, both their results and our own findings indicate better prefrontal functioning in Met allele
carriers, whereat two different correlates of prefrontal brain function were used (electrophysiologically assessed prefrontal noise _vs_ ERP components related to prefrontal inhibitory
control). Regarding the psychopathological data, patients homozygous for the Met allele tended to be more severely ill, which is in accordance with previous studies (Bilder et al, 2002). For
two of the three neuropsychological tests of frontal lobe function, no influence of _COMT_ genotype was found, whereas for the Stroop Test, a statistical trend indicated prolonged
interference times in patients carrying Val/Met as compared to the matched group of Met/Met patients. As both the Stroop interference condition (Gruber et al, 2002) and the NGA (Fallgatter
et al, 2002) have been associated with ACC functioning and our two groups of patients differed regarding their mean NGA, an accompanying difference in Stroop interference performance is
highly plausible. As the Stroop task was the only task we used that is thought to specifically involve the ACC, a missing effect in the other two tests—which have been shown to
preferentially activate other frontal areas such as the dorsolateral prefrontal cortex or Broca's area (Gaillard et al, 2000; Moll et al, 2002)—appears to be plausible as well. Based on
the electrophysiological findings, one might expect Val allele carriers to display an increased CPT error rate, particularly during Nogo trials (ie commission errors). The reason for the
absence of such a finding might well be the paradigm used: The version of the CPT employed in the present study was adapted for application in psychiatric samples, that is, with low
difficulty to avoid high error rates, making it less sensitive for respective performance differences. When interpreting the present findings, it has to be considered that the patient sample
was diagnostically heterogeneous, as not only patients with narrow-definition schizophrenia but also some patients with schizophreniform or schizoaffective disorders were included. Although
these diagnoses were equally distributed across the different genotype groups (see Patients and methods) so that the observed group differences cannot be attributed to diagnostic
between-group differences, it cannot be ruled out that the impact of _COMT_ genotype on prefrontal brain function would be different in a homogenous sample. Future studies with more
homogenous patient samples are therefore required; considering, however, that schizophrenia in itself is heterogeneous, and that many genetic studies investigate broad-spectrum schizophrenia
with greater success than narrow-spectrum schizophrenia, it is unlikely that those studies would yield differing results. In conclusion, the present data show a strong impact of _COMT_
genotype on an electrophysiological correlate of cognitive response control and prefrontal functioning (NGA) in a group of patients with schizophrenia spectrum disorders. Carriers of the
_COMT_ allele related to a particularly high activity of the enzyme and thus shorter availability of dopamine in the synaptic cleft (Val) showed a significantly diminished NGA, indications
of reduced fronto-central Nogo amplitudes, and an impaired Stroop performance, suggesting impaired functional activation of prefrontal structures probably including the ACC. These findings
thus verify the impact of COMT Val158Met on prefrontal functioning and confirm the relevance of prefrontal dopaminergic tone for cognition. As the NGA was virtually absent in Val allele
carriers, this might correspond to a genetically driven endophenotype in schizophrenic illnesses, possibly contributing to the cognitive deficits in schizophrenia. Strong genetic influences
on prefrontal functioning might also account for the partly inconsistent results regarding a specifically frontal pathology (‘hypofrontality’) in schizophrenias, that is, for the highly
variable symptomatology of disorders from the schizophrenic spectrum. Even though the present results need to be replicated in a larger sample and should also be confirmed by neuroimaging
methods, neurophysiological approaches such as the present one are valuable tools in attempts to further elucidate the impact of genetic variations on cognitive functioning and functional
brain activation in healthy subjects and neuropsychiatric disorders. REFERENCES * Abi-Dargham A, Moore H (2003). Prefrontal DA transmission at D1 receptors and the pathology of
schizophrenia. _Neuroscientist_ 9: 404–416. Article CAS PubMed Google Scholar * Andreasen NC, Rezai K, Alliger R, Swayze VW, Flaum M, Kirchner P _et al_ (1992). Hypofrontality in
neuroleptic-naïve patients and in patients with chronic schizophrenia. Assessment with xenon 133 single-photon emission computed tomography and the Tower of London. _Arch Gen Psychiatry_ 49:
943–958. Article CAS PubMed Google Scholar * Badner JA, Gershon ES (2002). Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. _Mol Psychiatry_ 7:
405–411. Article CAS PubMed Google Scholar * Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X _et al_ (2002). Neurocognitive correlates of the COMT Val(158)Met polymorphism
in chronic schizophrenia. _Biol Psychiatry_ 52: 701–707. Article CAS PubMed Google Scholar * Bokura H, Yamaguchi S, Kobayashi S (2001). Electrophysiological correlates for response
inhibition in a Go/NoGo task. _Clin Neurophysiol_ 112: 2224–2232. Article CAS PubMed Google Scholar * Carter CS, MacDonald AW, Ross LL, Stenger VA (2001). Anterior cingulate cortex
activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. _Am J Psychiatry_ 158: 1423–1428. Article CAS PubMed Google Scholar *
Carter CS, Mintun M, Nichols T, Cohen JD (1997). Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task
performance. _Am J Psychiatry_ 154: 1670–1675. Article CAS PubMed Google Scholar * Chen X, Wang X, O'Neill AF, Walsh D, Kendler KS (2004). Variants in the
catechol-_o_-methyltranserase (COMT) gene are associated with schizophrenia in Irish high-density families. _Mol Psychiatry_ 9: 962–967. Article CAS PubMed Google Scholar * Craddock N,
Owen MJ, O'Donovan MC (2006). The catechol-_O_-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessons. _Mol Psychiatry_ 11: 446–458. Article CAS
PubMed Google Scholar * de Zubicaray GI, Zelaya FO, Andrew C, Williams SC, Bullmore ET (2000). Cerebral regions associated with verbal response initiation, suppression and strategy use.
_Neuropsychologia_ 38: 1292–1304. Article CAS PubMed Google Scholar * Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE _et al_ (2001). Effect of COMT
Val108/158Met genotype on frontal lobe function and risk for schizophrenia. _Proc Natl Acad Sci USA_ 98: 6917–6922. Article CAS PubMed PubMed Central Google Scholar * Fallgatter AJ
(2001). Electrophysiology of the prefrontal cortex in healthy controls and schizophrenic patients: a review. _J Neural Transm_ 108: 679–694. Article CAS PubMed Google Scholar *
Fallgatter AJ, Bartsch AJ, Herrmann MJ (2002). Electrophysiological measurements of anterior cingulate function. _J Neural Transm_ 109: 977–988. Article CAS PubMed Google Scholar *
Fallgatter AJ, Bartsch AJ, Zielasek J, Herrmann MJ (2003). Brain electrical dysfunction of the anterior cingulate in schizophrenic patients. _Psychiatry Res_ 124: 37–48. Article PubMed
Google Scholar * Fallgatter AJ, Brandeis D, Strik WK (1997). A robust assessment of the NoGo-anteriorisation of P300 microstates in a cued Continuous Performance Test. _Brain Topogr_ 9:
295–302. Article CAS PubMed Google Scholar * Fallgatter AJ, Herrmann MJ, Roemmler J, Ehlis A-C, Wagener A, Heidrich A _et al_ (2004). Allelic variation of serotonin transporter function
modulates the brain electrical response for error processing. _Neuropsychopharmacology_ 29: 1506–1511. Article CAS PubMed Google Scholar * Fallgatter AJ, Mueller TJ (2001).
Electrophysiological signs of reduced prefrontal response control in schizophrenic patients. _Psychiatry Res Neuroimaging_ 107: 19–28. Article CAS Google Scholar * Ford JM, Gray M,
Whitfield SL, Turken AU, Glover G, Faustman WO _et al_ (2004). Acquiring and inhibiting prepotent responses in schizophrenia: event-related brain potentials and functional magnetic resonance
imaging. _Arch Gen Psychiatry_ 61: 119–129. Article PubMed Google Scholar * Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH (2000). Functional anatomy of
cognitive development: fMRI of verbal fluency in children and adults. _Neurology_ 54: 180–185. Article CAS PubMed Google Scholar * Gallinat J, Bajbouj M, Sander T, Schlattmann P, Xu K,
Ferro EF _et al_ (2003). Association of the G1947A COMT (Val(108/158)Met) gene polymorphism with prefrontal P300 during information processing. _Biol Psychiatry_ 54: 40–48. Article CAS
PubMed Google Scholar * Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS _et al_ (2003). Executive subprocesses in working memory: relationship to
catechol-_O_-methyltransferase Val158Met genotype and schizophrenia. _Arch Gen Psychiatry_ 60: 889–896. Article CAS PubMed Google Scholar * Gruber SA, Rogowska J, Holcomb P, Soraci S,
Yurgelun-Todd D (2002). Stroop performance in normal control subjects: an fMRI study. _Neuroimage_ 16: 349–360. Article PubMed Google Scholar * Hamilton M (1960). A rating scale for
depression. _J Neurol Neurosurg Psychiatry_ 23: 56–62. Article CAS PubMed PubMed Central Google Scholar * Inada T, Nakamura A, Iijima Y (2003). Relationship between
catechol-_O_-methyltransferase polymorphism and treatment-resistant schizophrenia. _Am J Med Genet_ 120B: 35–39. Article PubMed Google Scholar * Ingvar DH, Franzen G (1974). Abnormalities
of cerebral blood flow distribution in patients with chronic schizophrenia. _Acta Psychiatr Scand_ 50: 425–462. Article CAS PubMed Google Scholar * Jasper H (1958). Report of committee
on methods of clinical exam in EEG. _Electroencephalogr Clin Neurophysiol_ 10: 370–375. Article Google Scholar * Kay SR (1991). _Positive and Negative Symptoms in Schizophrenia_.
Brunner/Mazel: New York. Google Scholar * Kiehl KA, Smith AM, Hare RD, Liddle PF (2000). An event-related potential investigation of response inhibition in schizophrenia and psychopathy.
_Biol Psychiatry_ 48: 210–221. Article CAS PubMed Google Scholar * Kneavel M, Gogos J, Karayiorgou K, Luine V (2000). Interaction of COMT gene deletion and environment on cognition. _Soc
Neurosci Abstr_ 26: 571.20. Google Scholar * Kopp B, Rist F (1999). An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. _J
Abnorm Psychol_ 108: 337–346. Article CAS PubMed Google Scholar * Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF (2003). Rostral anterior cingulate cortex dysfunction during error
processing in schizophrenia. _Brain_ 126: 610–622. Article PubMed Google Scholar * Lehmann D (1987). Principles of spatial analysis. In: Gevins A, Remond A (eds). _Methods of Analysis of
Brain Electrical and Magnetic Signals. Handbook of Electroencephalography and Clinical Neurophysiology_. Elsevier: Amsterdam. pp 309–354. Google Scholar * Lehmann D, Skrandies W (1980).
Reference-free identification of components of checkerboard-evoked multichannel potential fields. _Electroencephalogr Clin Neurophysiol_ 48: 609–621. Article CAS PubMed Google Scholar *
Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM (2002). Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. _J Abnorm Psychol_ 111: 22–41.
Article PubMed Google Scholar * Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE (2004). Functional subdivisions within anterior cingulate cortex and their relationship to
autonomic nervous system function. _Neuroimage_ 22: 1151–1156. Article PubMed Google Scholar * Moll J, de Oliveira-Souza R, Moll FT, Bramati IE, Andreiuolo PA (2002). The cerebral
correlates of set-shifting: an fMRI study of the trail making test. _Arq Neuropsiquiatr_ 60: 900–905. Article PubMed Google Scholar * Nolan KA, Bilder RM, Lachman HM, Volavka J (2004).
Catechol _O_-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. _Am J Psychiatry_ 161: 359–361.
Article PubMed Google Scholar * Overall JE, Gorham DR (1962). The brief psychiatric rating scale. _Psychol Rev_ 10: 799–812. Google Scholar * Pascual-Marqui RD, Michel CM, Lehmann D
(1994). Low resolution electromagnetic tomography, a new method for localizing electrical activity in the brain. _Int J Psychophysiol_ 18: 49–65. Article CAS PubMed Google Scholar * Reif
A, Fallgatter AJ, Ehlis A-C, Lesch K-P (2004). Altered cingulate functioning in a case of chromosome 22q11 deletion syndrome. _Psychiat Res Neuroimaging_ 132: 273–278. Article Google
Scholar * Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A _et al_ (2001). An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory
function. _Schizophr Res_ 52: 47–55. Article CAS PubMed Google Scholar * Sanders AR, Rusu I, Duan J, Vander Molen JE, Hou C, Schwab SG _et al_ (2005). Haplotypic association spanning the
22q11.21 genes COMT and ARVCF with schizophrenia. _Mol Psychiatry_ 10: 353–365. Article CAS PubMed Google Scholar * Strandburg RJ, Marsh JT, Brown WS, Asarnow RF, Guthrie D, Harper R
_et al_ (1999). Continuous-processing related ERPs in adult schizophrenia: continuity with childhood onset schizophrenia. _Biol Psychiatry_ 45: 1356–1369. Article CAS PubMed Google
Scholar * Strik WK, Fallgatter AJ, Brandeis D, Pascual-Marqui R (1998). Three dimensional tomography of event-related potentials during response inhibition: evidence for phasic frontal lobe
activation. _Electroencephalogr Clin Neurophysiol_ 108: 406–413. Article CAS PubMed Google Scholar * Sullivan PF, Kendler KS, Neale MC (2003). Schizophrenia as a complex trait: evidence
from a meta-analysis of twin studies. _Arch Gen Psychiatry_ 60: 1187–1192. Article PubMed Google Scholar * Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN _et al_
(1992). Limbic system abnormalities identified in schizophrenia with fluorodeoxyglucose and neocortical alterations with the deficit syndrome. _Arch Gen Psychiatry_ 49: 522–530. Article CAS
PubMed Google Scholar * Tsai S-J, Yu YW-Y, Chen T-J, Chen J-Y, Liou Y-J, Chen M-C _et al_ (2003). Association study of a functional catechol-_O_-methyltransferase-gene polymorphism and
cognitive function in healthy females. _Neurosci Lett_ 338: 123–126. Article CAS PubMed Google Scholar * Tunbridge EM, Harrison PJ, Weinberger DR (2006). Catechol-o-methyltransferase,
cognition, and psychosis: Val158Met and beyond. _Biol Psychiatry_, print copy in press (originally published online Feb. 14, 2006, at www.sciencedirect.com). Article CAS PubMed Google
Scholar * Volz H, Gaser C, Hager F, Rzanny R, Ponisch J, Mentzel H _et al_ (1999). Decreased frontal activation in schizophrenics during stimulation with the continuous performance test—a
functional magnetic resonance imaging study. _Eur Psychiatry_ 14: 17–24. Article CAS PubMed Google Scholar * Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK _et
al_ (2001). Prefrontal neurons and the genetics of schizophrenia. _Biol Psychiatry_ 50: 825–844. Article CAS PubMed Google Scholar * Weisbrod M, Kiefer M, Marzinzik F, Spitzer M (2000).
Executive control is disturbed in schizophrenia: evidence from event-related potentials in a Go/NoGo task. _Biol Psychiatry_ 47: 51–60. Article CAS PubMed Google Scholar * Wonodi I,
Stine OC, Mitchell BD, Buchanan RW, Thaker GK (2003). Association between Val108/158Met polymorphism of the _COMT_ gene and schizophrenia. _Am J Med Genet_ 120B: 47–50. Article PubMed
Google Scholar Download references ACKNOWLEDGEMENTS We gratefully acknowledge Theresa Töpner, Inge Gröbner, and Melanie Harder for excellent technical assistance. This study was supported
by the Deutsche Forschungsgemeinschaft (Grant RE1632/1-1 to AR, KFO 125/1-1 to AR, AJF, and KPL, SFB 581 to KPL, and FA 361/8-1+2 to AJF) and Bundesministerium für Bildung, Wissenschaft,
Forschung und Technologie (IZKF, 01KS9603 to KPL). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Psychiatry and Psychotherapy, University of Wuerzburg, Wuerzburg, Germany
Ann-Christine Ehlis, Andreas Reif, Martin J Herrmann, Klaus-Peter Lesch & Andreas J Fallgatter Authors * Ann-Christine Ehlis View author publications You can also search for this author
inPubMed Google Scholar * Andreas Reif View author publications You can also search for this author inPubMed Google Scholar * Martin J Herrmann View author publications You can also search
for this author inPubMed Google Scholar * Klaus-Peter Lesch View author publications You can also search for this author inPubMed Google Scholar * Andreas J Fallgatter View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Ann-Christine Ehlis. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Ehlis, AC., Reif, A., Herrmann, M. _et al._ Impact of Catechol-_O_-Methyltransferase on Prefrontal Brain Functioning in Schizophrenia Spectrum Disorders.
_Neuropsychopharmacol_ 32, 162–170 (2007). https://doi.org/10.1038/sj.npp.1301151 Download citation * Received: 28 November 2005 * Revised: 13 April 2006 * Accepted: 17 May 2006 * Published:
28 June 2006 * Issue Date: 01 January 2007 * DOI: https://doi.org/10.1038/sj.npp.1301151 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * COMT
(catechol-_O_-methyltransferase) * schizophrenia * prefrontal cortex * anterior cingulate cortex (ACC) * response control * executive functions