DNp73α protects myogenic cells from apoptosis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
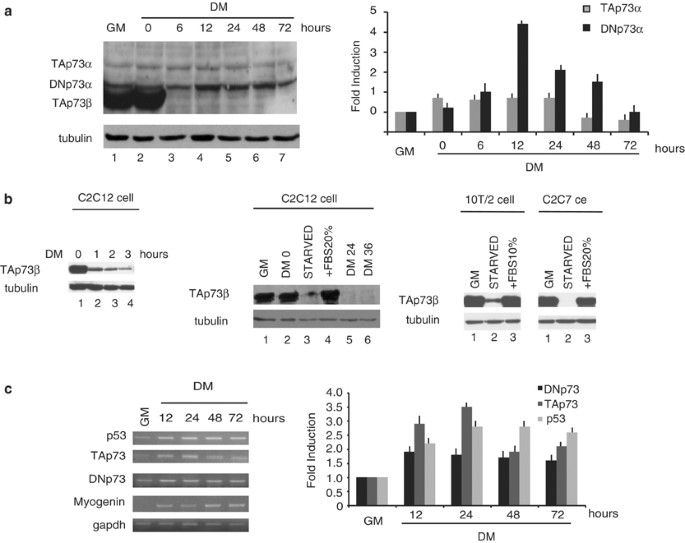
The P73 gene is transcribed from two promoters, P1 and P2, that direct the expression of multiple transactivation competent (TA) and dominant negative (DN) isoforms. TAp73 transcription
factors mediate cell cycle arrest and/or apoptosis in response to DNA damage and are involved in developmental processes. P73 mRNA levels increase and the P1p73 promoter is upregulated
during myogenic differentiation of C2C12 skeletal muscle satellite cells. The DNp73 proteins act as trans-repressors of p53- and p73-dependent transcription, and possess both antiapoptotic
and pro-proliferative potential. Here, we show that DNp73α is expressed in proliferating C2C12 myoblasts, rapidly accumulates in differentiating myocytes and remains elevated in C2C12
myotubes. By combining transactivation assays and chromatin immunoprecipitation analysis, we could show that the upregulation of the P2p73 promoter during myogenic differentiation is
mediated by the coordinated recruitment and activity of MyoD and p53/p73. Abrogation of DNp73 expression by specific siRNA led to a strong potentiation of the spontaneous apoptosis of C2C12
myoblasts induced to differentiate. Finally, unlike TAp73 that contributes to DNA damage-induced apoptosis of myotubes, endogenous DNp73 mediates the relative resistance of differentiated
myotubes to DNA damage. Altogether, our findings identify DNp73α as an important target in designing strategies aimed at the potentiation of the regenerative potential of skeletal satellite
cells.
This work was supported by grants from AIRC and MIUR-FIRB (to ML and GB), European Community (LSHC-CT-2004-503576 to ML and GB), Telethon (To GB and ML) and MIUR-Cofin and Schering-Plough
(to ML). LB and PM were supported by a fellowship from the Fondazione A. Cesalpino. FM was supported by a fellowship from FIRC. AD was supported by a fellowship from EMBO.
Fondazione A Cesalpino and Department of Internal Medicine, University of Rome ‘La Sapienza’, Rome, Italy
Rome Oncogenomic Center, Regina Elena Cancer Institute, Rome, Italy
Department of Experimental Oncology, Regina Elena Cancer Center, Rome, Italy
Department of Dermatology, University of Tor Vergata, Rome, Italy
Anyone you share the following link with will be able to read this content: