Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
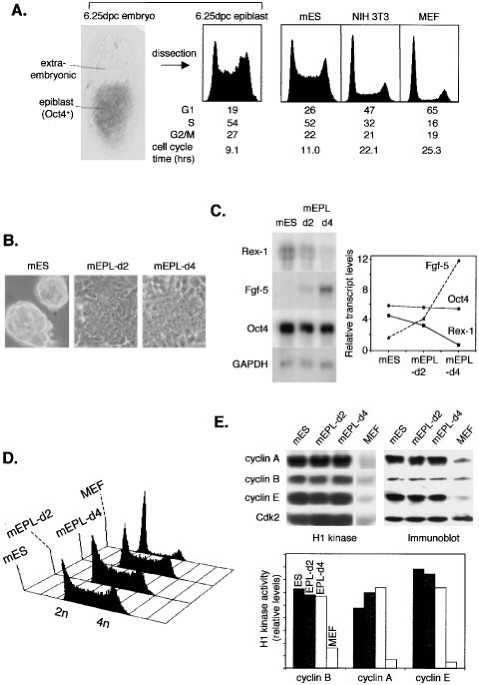
Pluripotent cells of embryonic origin proliferate at unusually rapid rates and have a characteristic cell cycle structure with truncated gap phases. To define the molecular basis for this we
have characterized the cell cycle control of murine embryonic stem cells and early primitive ectoderm-like cells. These cells display precocious Cdk2, cyclin A and cyclin E kinase
activities that are conspicuously cell cycle independent. Suppression of Cdk2 activity significantly decreased cycling times of pluripotent cells, indicating it to be rate-limiting for rapid
cell division, although this had no impact on cell cycle structure and the establishment of extended gap phases. Cdc2-cyclin B was the only Cdk activity that was identified to be cell cycle
regulated in pluripotent cells. Cell cycle regulation of cyclin B levels and Y15 regulation of Cdc2 contribute to the temporal changes in Cdc2-cyclin B activity. E2F target genes are
constitutively active throughout the cell cycle, reflecting the low activity of pocket proteins such as p107 and pRb and constitutive activity of pRb-kinases. These results show that rapid
cell division cycles in primitive cells of embryonic origin are driven by extreme levels of Cdk activity that lack normal cell cycle periodicity.
E Stead and J White contributed equally to this work. We thank Peter Wovkulich for technical assistance, Brian Gabrielli for many useful discussions, Peter Cartwright for critical reading of
the manuscript and Brian Gabrielli for the GST-Cdc25b expression construct. This work was supported by grants from the National Health and Medical Research Council (NHMRC) of Australia and
from the Australian Research Council (ARC) through the Centre for Molecular Genetics of Development. E Stead and J White were supported by Australian Post-Graduate Research Awards.
Department of Molecular Biosciences and Center for Molecular Genetics of Development, University of Adelaide, North Terrace, Adelaide, 5005, South Australia, Australia
Elaine Stead, Josephine White, Renate Faast, Simon Conn, Sherilyn Goldstone, Joy Rathjen, Peter Rathjen & Stephen Dalton
BresaGen-Luminis Cell Therapy Program, University of Adelaide, North Terrace, Adelaide, 5005, South Australia, Australia
Hoffman La Roche, 340 Kingsland Street, Nutley, 07110, New Jersey, NJ, USA
Anyone you share the following link with will be able to read this content: