COVID-19 At-Home Tests Sold at CVS, Amazon Recalled
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Facebook Twitter LinkedIn
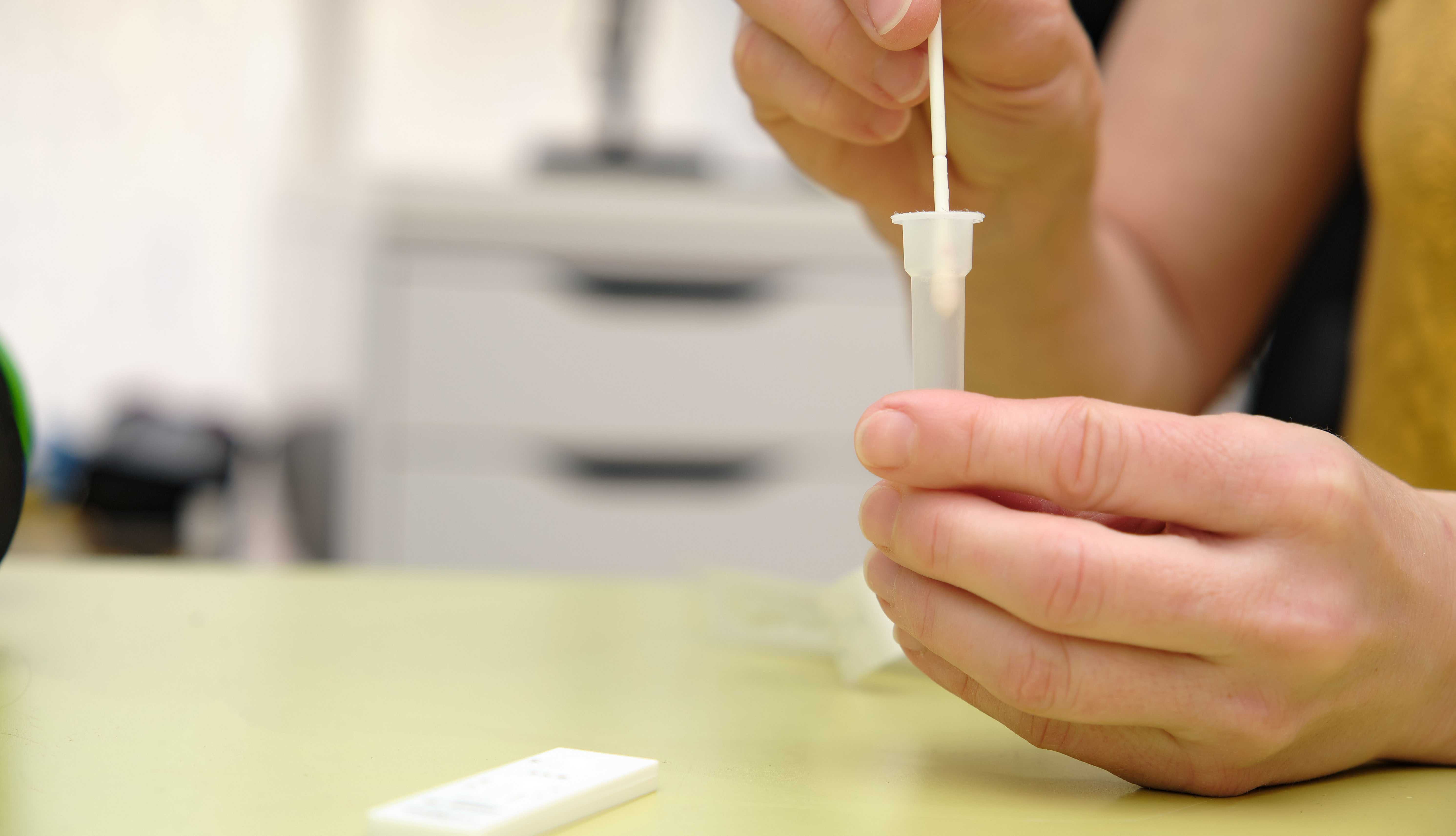
The U.S. Food and Drug Administration (FDA) is warning consumers and health providers to stop using and toss out certain Pilot COVID-19 At-Home tests sold at CVS and Amazon due to
“significant concerns” of bacterial contamination.
Manufacturer SD Biosensor is recalling some 500,000 tests distributed to CVS Health and 16,000 tests distributed to Amazon by Roche Diagnostics. The FDA and Roche Diagnostics are working to
determine how many of the kits were sold to consumers.
Courtesy of SD Biosensor, Inc.None of the contaminated tests were distributed through the government’s free COVID test programs. If you received your tests via COVID.gov/tests, or as part of a federal testing program,
they are not subject to the recall.
The FDA issued an advisory saying there are concerns the liquid solution provided with the kit was contaminated with bacterias such as enterococcus, enterobacter, klebsiella and serratia
species and that direct contact with it could cause an infection.
In particular, FDA said these types of bacteria may cause illness in people with weakened immune systems or those with direct exposure to the contaminated liquid solution through standard
handling, accidental spills, or misuse of the product. What’s more, the bacterial contamination could impact the accuracy of the test.
If you have one of the contaminated kits, the FDA said to throw it in the trash and to avoid flushing the liquid solution down the drain. If the liquid in the tube contacts your skin and
eyes, flush with large amounts of water. If irritation persists, the FDA said to seek medical attention.
Be on the lookout for signs of a bacterial infection which include fever, discharge, and red eyes. If those are present or any other concerning symptoms arise, contact your doctor. If you
have concerns about the result of one of these tests, talk to your healthcare provider.
If you think you had a problem with the SD Biosensor Pilot COVID-19 At-Home Test, the FDA encourages you to report the problem through the MedWatch Voluntary Reporting Form.
RecalledLots
The FDA is advising consumers to stop using and throw out Pilot COVID-19 At-Home Test kits with the following lot numbers:
53K38N1T1 53K4221T1 53K4292T1 53K38N2T153K4222T153K42A1T153K38N3T153K4223T153K42A2T153K38N4T153K4224T153K42A3T153K38N5T153K4225T153K42E1T153K38P1T153K4231T153K42G1T153K38P2T153K4232T153K42G2T153K38P3T153K4233T153K42H1T153K41T5T153K4261T153K42H2T153K41X1T153K4262T153K42L1T153K41X2T53K4271T153K42L2T153K41X3T153K4272T1
53K4361AC53K4211T153K4273T153K4362AC53K4212T153K4274T153K4392AC53K4213T153K4291T1 %{postComment}%
Donna Fuscaldo is a contributing writer and editor focusing on personal finance and health. She has spent over two decades writing and covering news for several national publications
including The Wall Street Journal, Forbes, Investopedia and HerMoney.
Unlock Access to AARP Members Edition
Join AARP to Continue Already a Member? Login
AARP NEWSLETTERS
%{ newsLetterPromoText }%
%{ description }%
Subscribe See All NewslettersPrivacy Hub