Intravesicular administration of sodium hyaluronate ameliorates the inflammation and cell proliferation of cystitis cystica et glandularis involving interleukin-6/JAK2/Stat3 signaling pathway
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Cystitis cystica et glandularis (CCEG) is a chronic cystitis that causes extreme agony in affected patients. However, there are lack of effective conservative treatments. In this study, it
is evident that intravesicular sodium hyaluronate (SH) therapy significantly improved the clinical symptoms of CCEG patients and ameliorated the bladder mucosal inflammation and cell
proliferation characteristics of the disease. Immunohistochemical staining showed that the staining intensities of hyaluronidase (HYAL 1/2), CD44, IL-6 and phosphorylated signal transducer
and activator of transcription 3 (p-Stat3) in bladder mucosal tissue were significantly increased in CCEG patients compared with control patients and that intravesicular SH treatment
suppressed these protein expression. We established a CCEG rat model by treating rats with E. coli intravesicularly, and we found that HYAL 1/2 and CD44 expression levels were significantly
increased in the E. coli group compared with the NC group. Activation of the IL-6/JAK2/Stat3 pathway and the expression levels of the downstream pro-apoptotic proteins Mcl-1 and Bcl-xL were
also significantly increased in the E. coli group compared with the NC group. The above changes were significantly mitigated by intravesicular SH treatment. Therefore, SH may serve as an
effective therapy for CCEG by inhibiting bladder mucosal inflammation and proliferation.
Cystitis cystica et glandularis (CCEG) is a chronic reactive inflammatory disorder thought to be attributable to chronic urothelial irritation caused by infections, tumors, calculi or outlet
obstructions1. CCEG is characterized by pathologic proliferative changes in the bladder mucosa. Early studies suggest that CCEG is a precancerous lesion2,3. Recently, more and more
evidences suggest that there is no significant causal relationship between CCEG and bladder malignancies, but there exists phenomenon of coexistence of cystitis glandularis and bladder
carcinoma with high ratio4,5,6.
The typical symptoms of CCEG are urinary frequency, urinary urgency, dysuria, and hematuria, which cause extreme discomfort in affected patients and reduce their quality of life.
Improvements in cystoscopy and biopsy techniques have led to a gradual increase in the number of reports about CCEG over the last decade. However, the pathogenesis of CCEG remains unclear7.
Given that CCEG is a chronic reactive inflammatory disorder that causes pathologic proliferative changes in the bladder mucosa, we elected to focus on the mechanisms responsible for the
inflammation and cell proliferation characteristic of CCEG in this study.
Increasing amounts of evidence have indicated that inflammatory signals play an important role in sustaining and promoting neoplastic growth. The pro-inflammatory cytokine IL-6 and its
downstream effectors, Janus-activated kinases (JAK2) and signal transducer and activator of transcription 3 (Stat3), have been demonstrated to play important roles in blocking cell apoptosis
and enhancing cell proliferation in various hyperplastic diseases8,9. For example, IL-6 has been reported to induce phosphorylation of Stat3, which is associated with increases in the
expression levels of the anti-apoptotic genes Bcl-xL and Mcl-1 in Barrett’s esophagus, a condition that appears to result from chronic irritation and is characterized by the replacement of
dysplastic squamous epithelial cells with metaplastic intestinal-like columnar epithelial cells10,11. Similar pathologic changes occur in the bladder in CCEG. The IL-6/JAK2/Stat3 pathway
plays a critical role in inflammation and proliferation. Both of inflammation and proliferation occur in the bladder mucosa in CCEG. Thus, it is hypothesised that the IL-6/JAK2/Stat3 pathway
is also involved in CEGG development and progression. Thus, in this study, we investigated the activity of the IL-6/JAK2/Stat3 signaling pathway and assessed the expression of the
downstream anti-apoptotic biomarkers Mcl-1 and Bcl-xL to elucidate the molecular mechanisms underlying the development of CCEG.
Glycosaminoglycans (GAGs) form a thick layer that covers the bladder epithelium to block various irritants, such as chemicals, calculi and bacteria that cause chronic infections12. The
protection provided by GAG layers may prevent the constant evolution of bladder inflammation. GAGs have recently become a novel therapy for the treatment of recurrent urinary tract
infections and interstitial cystitis/painful bladder syndrome (IC/PBS)13,14,15. Endogenous hyaluronic acid (HA) is a key component of GAGs, and recent studies have suggested that
intravesicular instillation of sodium hyaluronate (SH) may promote regeneration of the GAG layers on the bladder urothelium and inhibit IL-6 secretion in bladder tissue16. However, few
studies have investigated the effects of SH treatment on human CCEG. Thus, in the present study, we evaluated the effects of treatment with SH on CCEG patients and elucidated the mechanisms
linking the IL-6/JAK2/Stat3 pathway to CCEG in a CCEG rat model.
All CCEG patients were given pre- and post-treatment “PUF Patient Symptom Scale Questionnaires” to assess the effects of treatment with SH on their clinical symptoms. The voiding dairies
completed by the patients enrolled herein were used to estimate their mean daytime urinary frequency and their maximum bladder volume. After SH treatment, CCEG patients displayed significant
improvements in their bladder pain, daytime urinary frequency and maximum bladder volumes (Table 1). With the exception of one patient who experienced two episodes of transient whole-body
itching, no patients experienced severe adverse events during the indicated period.
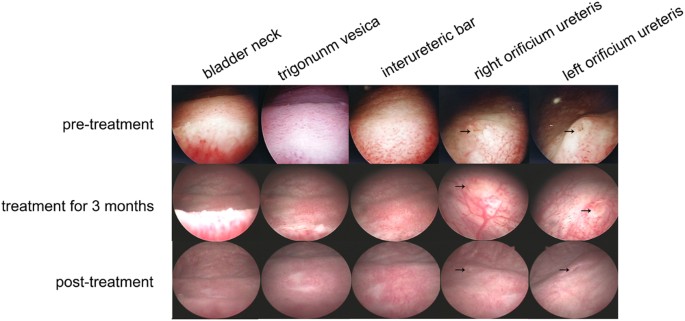
In the control group, the mucosa of the bladder trigonum and bladder neck appeared pink and smooth under 70° cystoscopy. In 16 CCEG patients, the bladder trigonum and bladder neck displayed
white villous, follicular, papillary changes before SH treatment. Eleven of the indicated patients had inflammatory congestion. All CCEG patients experienced varying degrees of improvement
in the appearance of their bladder mucosal tissues after being treated with SH for 6 months, and 6 patients displayed normal bladder mucosa under cystoscopy, i.e., mucosa that appeared pink
and smooth under cystoscopy, after treatment with SH (Fig. 1).
Cystoscopy showed improvement of bladder mucosal lesions in CCEG patients at different time points during SH treatment. The arrows indicate the orificium ureteris.
HE staining showed that patients in the control group had normal bladder mucosal tissues with an intact epithelial layer and that no pathological changes had occurred, and no inflammatory
cell infiltrates were present in the submucosa. Severe epithelial damage, Brunn’s nests, cysts and inflammatory cell infiltrates were observed in the submucosa in all the samples obtained
from patients in the pre-treatment group. Treatment with SH significantly ameliorated CCEG-induced inflammation and cell proliferation in the bladder mucosa in the post-treatment group. The
patients in this group were found to have an intact epithelial layer and decreased numbers of Brunn’s nests and cysts and less severe inflammatory cell infiltration compared with their
counterparts in the pre-treatment group (Fig. 2). The data pertaining to patient histological scores, inflammatory cell counts and Brunn’s nest counts demonstrated that CCEG induced severe
inflammation and significant cell proliferation in the pre-treatment group and that these changes were significantly ameliorated in the post-treatment group (Table 1).
Representative histological images of HE staining showed that SH treatment ameliorated bladder mucosal inflammation and cell proliferation for CCEG patients.
Under pathological conditions, internalized HA is degraded by hyaluronidases (such as HYAL 1/2)17, and the products of this degradation have been demonstrated to mediate extensive
inflammatory responses by interacting with the CD44 receptor18,19,20. We detected the HYAL 1/2 and CD44 expression and localization in human samples using the semi-quantitative IHC analysis.
The results showed that the HYAL 1/2 and CD44 were localized in urothelial cells and their staining intensities were significantly increased in the pre-treatment group compared with the
control group. After SH treatment, the staining intensities of HYAL 1/2 and CD44 were significantly decreased in the post-treatment group (Fig. 3).
Representative images of IHC staining for HYAL-1, HYAL-2 and CD44 in the bladder mucosa (bar = 100 μm) and comparison of H-scores among the three groups. Values are expressed as mean ± SEM,
n = 16 in pre-treatment group and post-treatment group, n = 6 in normal control group. *P