Medial preoptic circuit induces hunting-like actions to target objects and prey
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT As animals forage, they must obtain useful targets by orchestrating appropriate actions that range from searching to chasing, biting and carrying. Here, we reveal that neurons
positive for the α subunit of Ca2+/calmodulin-dependent kinase II (CaMKIIα) in the medial preoptic area (MPA) that send projections to the ventral periaqueductal gray (vPAG) mediate these
target-directed actions in mice. During photostimulation of the MPA–vPAG circuit, mice vigorously engaged with 3D objects and chased moving objects. When exposed to a cricket, they hunted
down the prey and bit it to kill. By applying a head-mounted object control with timely photostimulation of the MPA–vPAG circuit, we found that MPA–vPAG circuit-induced actions occurred only
when the target was detected within the binocular visual field. Using this device, we successfully guided mice to navigate specified routes. Our study explains how the brain yields a strong
motivation to acquire a target object along the continuum of hunting behavior. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel
any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS PERIAQUEDUCTAL GRAY NEURONS ENCODE THE SEQUENTIAL MOTOR PROGRAM IN HUNTING BEHAVIOR OF MICE
Article Open access 11 November 2021 A CIRCUIT FROM THE VENTRAL SUBICULUM TO ANTERIOR HYPOTHALAMIC NUCLEUS GABAERGIC NEURONS ESSENTIAL FOR ANXIETY-LIKE BEHAVIORAL AVOIDANCE Article Open
access 03 December 2022 THE TECTONIGRAL PATHWAY REGULATES APPETITIVE LOCOMOTION IN PREDATORY HUNTING IN MICE Article Open access 20 July 2021 DATA AVAILABILITY The data that support the
findings of this study are available from the corresponding author upon reasonable request. CHANGE HISTORY * _ 07 MARCH 2018 In the version of this article initially published, a sentence in
the fifth paragraph of the Results read, “Immunohistochemistry revealed that VGLUT2+ MPA neurons rarely expressed CaMKIIα, which is a putative marker for subcortical glutamatergic neurons.”
It should have read, “Immunohistochemistry revealed that CaMKIIα+ MPA neurons rarely expressed VGLUT2, which is a putative marker for subcortical glutamatergic neurons.” The error has been
corrected in the HTML and PDF versions of the article. In the supplementary information originally posted online, the wrong version of Supplementary Fig. 1 was posted and some of the
supplementary videos were interchanged. In the corrected Supplementary Fig. 1, the top right subpanel was added and the original Supplementary Fig. 1a was divided into 1a and 1b, with
subsequent panels incremented accordingly. The legend was changed from “A. Schematic illustrating electrical lesioning of the rat anterior hypothalamus. Electrical lesion areas (gray) in
five representative brain sections are depicted. Scale bar, 1 mm” to “A. Repetitive electrical stimulations of the anterior hypothalamus using bipolar electrodes (_Left_) caused a lesion at
the hypothalamic area (_middle_, marked by asterisk) successfully in 7 rats (_Right_, overlapped images of brain sections located from the bregma −0.24 mm). Scale bar, 1 mm. B. Electrical
lesion areas (gray) in five representative brain sections from anterior to posterior are depicted.” The errors have been corrected online. _ REFERENCES * Inglis, I. R., Langton, S., Forkman,
B. & Lazarus, J. An information primacy model of exploratory and foraging behaviour. _Anim. Behav._ 62, 543–557 (2001). Article Google Scholar * Dunbar, R. I. Animal play behavior.
_Behav. Process._ 8, 98–101 (1983). https://doi.org/10.1016/0376-6357(83)90049-9. Article Google Scholar * Bateson, P. & Young, M. The influence of male kittens on the object play of
their female siblings. _Behav. Neural Biol._ 27, 374–378 (1979). Article CAS Google Scholar * Ferster, C. B. & Skinner, B. F. _Schedules of Reinforcement_ (B. F. Skinner Foundation,
1957). * Skinner, B. F. Operant behavior. _Am. Psychol._ 18, 503 (1963). Article Google Scholar * Klein, M. O. et al. Periaqueductal gray μ and κ opioid receptors determine behavioral
selection from maternal to predatory behavior in lactating rats. _Behav. Brain Res._ 274, 62–72 (2014). Article CAS Google Scholar * Mota-Ortiz, S. R. et al. The periaqueductal gray as a
critical site to mediate reward seeking during predatory hunting. _Behav. Brain Res._ 226, 32–40 (2012). Article Google Scholar * Comoli, E., Ribeiro-Barbosa, E. R. & Canteras, N. S.
Predatory hunting and exposure to a live predator induce opposite patterns of Fos immunoreactivity in the PAG. _Behav. Brain Res._ 138, 17–28 (2003). Article CAS Google Scholar * Han, W.
et al. Integrated control of predatory hunting by the central nucleus of the amygdala. _Cell_ 168, 311–324.e318 (2017). Article CAS Google Scholar * Beitz, A. J. The organization of
afferent projections to the midbrain periaqueductal gray of the rat. _Neuroscience_ 7, 133–159 (1982). Article CAS Google Scholar * Rizvi, T. A., Ennis, M. & Shipley, M. T. Reciprocal
connections between the medial preoptic area and the midbrain periaqueductal gray in rat: a WGA-HRP and PHA-L study. _J. Comp. Neurol._ 315, 1–15 (1992). Article CAS Google Scholar *
Malsbury, C. W. Facilitation of male rat copulatory behavior by electrical stimulation of the medial preoptic area. _Physiol. Behav._ 7, 797–805 (1971). Article CAS Google Scholar * Wu,
Z., Autry, A. E., Bergan, J. F., Watabe-Uchida, M. & Dulac, C. G. Galanin neurons in the medial preoptic area govern parental behaviour. _Nature_ 509, 325–330 (2014). Article CAS
Google Scholar * Silva, B. A. et al. Independent hypothalamic circuits for social and predator fear. _Nat. Neurosci._ 16, 1731–1733 (2013). Article CAS Google Scholar * Wang, L., Chen,
I. Z. & Lin, D. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. _Neuron_ 85, 1344–1358 (2015). Article CAS Google Scholar * Lin, D. et al.
Functional identification of an aggression locus in the mouse hypothalamus. _Nature_ 470, 221–226 (2011). Article CAS Google Scholar * Falkner, A. L., Grosenick, L., Davidson, T. J.,
Deisseroth, K. & Lin, D. Hypothalamic control of male aggression-seeking behavior. _Nat. Neurosci._ 19, 596–604 (2016). Article CAS Google Scholar * Falkner, A. L., Dollar, P.,
Perona, P., Anderson, D. J. & Lin, D. Decoding ventromedial hypothalamic neural activity during male mouse aggression. _J. Neurosci._ 34, 5971–5984 (2014). Article CAS Google Scholar
* Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. _Nat. Neurosci._ 14, 351–355 (2011). Article CAS
Google Scholar * Anand, B. K. & Brobeck, J. R. Localization of a “feeding center” in the hypothalamus of the rat. _Proc. Soc. Exp. Biol. Med._ 77, 323–324 (1951). Article CAS Google
Scholar * Davidson, J. M. Activation of the male rat’s sexual behavior by intracerebral implantation of androgen. _Endocrinology_ 79, 783–794 (1966). Article CAS Google Scholar * Lee, H.
et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. _Nature_ 509, 627–632 (2014). Article CAS Google Scholar * Wallace, R. J. Hoarding of
inedible objects by albino rats. _Behav. Biol._ 23, 409–414 (1978). Article Google Scholar * Wallace, R. J. Novelty and partibility as determinants of hoarding in the albino rat. _Anim.
Learn. Behav._ 7, 549–554 (1979). Article Google Scholar * Morgan, J. I., Cohen, D. R., Hempstead, J. L. & Curran, T. Mapping patterns of c-fos expression in the central nervous system
after seizure. _Science_ 237, 192–197 (1987). Article CAS Google Scholar * Kim, D., Chae, S., Lee, J., Yang, H. & Shin, H. S. Variations in the behaviors to novel objects among five
inbred strains of mice. _Genes. Brain Behav._ 4, 302–306 (2005). Article CAS Google Scholar * Zhang, F., Wang, L. P., Boyden, E. S. & Deisseroth, K. Channelrhodopsin-2 and optical
control of excitable cells. _Nat. Methods_ 3, 785–792 (2006). Article CAS Google Scholar * Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone
to POMC neurons. _Neuron_ 71, 142–154 (2011). Article CAS Google Scholar * Fremeau, R. T. Jr. et al. The expression of vesicular glutamate transporters defines two classes of excitatory
synapse. _Neuron_ 31, 247–260 (2001). Article CAS Google Scholar * Gorski, R. A., Harlan, R. E., Jacobson, C. D., Shryne, J. E. & Southam, A. M. Evidence for the existence of a
sexually dimorphic nucleus in the preoptic area of the rat. _J. Comp. Neurol._ 193, 529–539 (1980). Article CAS Google Scholar * Raisman, G. & Field, P. M. Sexual dimorphism in the
preoptic area of the rat. _Science_ 173, 731–733 (1971). Article CAS Google Scholar * Gunaydin, L. A. et al. Natural neural projection dynamics underlying social behavior. _Cell_ 157,
1535–1551 (2014). Article CAS Google Scholar * Heesy, C. P. On the relationship between orbit orientation and binocular visual field overlap in mammals. _Anat. Rec. A Discov. Mol. Cell.
Evol. Biol._ 281, 1104–1110 (2004). Article Google Scholar * Numan, M. Medial preoptic area and maternal behavior in the female rat. _J. Comp. Physiol. Psychol._ 87, 746–759 (1974).
Article CAS Google Scholar * Chung, S. et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. _Nature_ 545, 477–481 (2017). Article CAS Google
Scholar * Ziegler, D. R., Cullinan, W. E. & Herman, J. P. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. _J. Comp. Neurol._ 448, 217–229 (2002). Article CAS
Google Scholar * Kiss, J., Kocsis, K., Csáki, A. & Halász, B. Evidence for vesicular glutamate transporter synapses onto gonadotropin-releasing hormone and other neurons in the rat
medial preoptic area. _Eur. J. Neurosci._ 18, 3267–3278 (2003). Article CAS Google Scholar * Oka, T., Tsumori, T., Yokota, S. & Yasui, Y. Neuroanatomical and neurochemical
organization of projections from the central amygdaloid nucleus to the nucleus retroambiguus via the periaqueductal gray in the rat. _Neurosci. Res._ 62, 286–298 (2008). Article CAS Google
Scholar * Wilson, A. M. et al. Locomotion dynamics of hunting in wild cheetahs. _Nature_ 498, 185–189 (2013). Article CAS Google Scholar * Paul, L. Predatory attack by rats: its
relationship to feeding and type of prey. _J. Comp. Physiol. Psychol._ 78, 69–76 (1972). Article CAS Google Scholar * Valone, T. J. & Lima, S. L. Carrying food items to cover for
consumption: the behavior of ten bird species feeding under the risk of predation. _Oecologia_ 71, 286–294 (1987). Article CAS Google Scholar * Talwar, S. K. et al. Rat navigation guided
by remote control. _Nature_ 417, 37–38 (2002). Article CAS Google Scholar * Maharbiz, M. & Sato, H. Cyborg beetles. _Sci. Am._ 303, 94–99 (2010). * O’Connell, L. A. & Hofmann, H.
A. Evolution of a vertebrate social decision-making network. _Science_ 336, 1154–1157 (2012). Article Google Scholar * Mueller, A. et al. Pathologisches Kaufen und psychische Komorbidität
[Compulsive buying and psychiatric comorbidity]. _Psychother. Psychosom. Med. Psychol._ 59, 291–299 (2009). Article Google Scholar * Grant, J. E. & Kim, S. W. Clinical characteristics
and associated psychopathology of 22 patients with kleptomania. _Compr. Psychiatr_ 43, 378–384, https://doi.org/10.1053/comp.2002.34628 (2002).. * Saxena, S. et al. Obsessive-compulsive
hoarding: symptom severity and response to multimodal treatment. _J. Clin. Psychiatry_ 63, 21–27 (2002). Article Google Scholar * Gray, P. The decline of play and the rise of
psychopathology in children and adolescents. _Am. J. Play._ 3, 443–463 (2011). Google Scholar * Paxinos, G. & Franklin, K.B.J. _The Mouse Brain in Stereotaxic Coordinates_ (Elsevier
Academic Press, 2008) * Piché, M., Robert, S., Miceli, D. & Bronchti, G. Environmental enrichment enhances auditory takeover of the occipital cortex in anophthalmic mice. _Eur. J.
Neurosci._ 20, 3463–3472 (2004). Article Google Scholar * Mendonça, D. F. et al. The inactive form of glycogen synthase kinase-3β is associated with the development of carcinomas in
galectin-3 wild-type mice, but not in galectin-3-deficient mice. _Int. J. Clin. Exp. Pathol._ 5, 547–554 (2012). PubMed PubMed Central Google Scholar * Cook-Snyder, D. R., Jones, A. &
Reijmers, L. G. A retrograde adeno-associated virus for collecting ribosome-bound mRNA from anatomically defined projection neurons. _Front. Mol. Neurosci._ 8, 56 (2015). Article Google
Scholar * Morrison, H. W. & Filosa, J. A. Sex differences in astrocyte and microglia responses immediately following middle cerebral artery occlusion in adult mice. _Neuroscience_ 339,
85–99 (2016). Article CAS Google Scholar * Musser, M. A., Correa, H. & Southard-Smith, E. M. Enteric neuron imbalance and proximal dysmotility in ganglionated intestine of the
Sox10Dom/+ Hirschsprung mouse model. _Cell. Mol. Gastroenterol. Hepatol._ 1, 87–101 (2015). Article Google Scholar * Cui, G. et al. Concurrent activation of striatal direct and indirect
pathways during action initiation. _Nature_ 494, 238–242 (2013). Article CAS Google Scholar * Kim, J. et al. Inhibitory basal ganglia inputs induce excitatory motor signals in the
thalamus. _Neuron_ 95, 1181–1196. e1188 (2017). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank K. Deisseroth at Stanford University for generously sharing the
channelrhodopsin vectors and Y-S. Jeong, J-E. Choi and M-K. Han for their assistance with experiments. We also thank to S. Park for assistance with writing. This work was supported by
Samsung Science and Technology Foundation under Project Number SSTF-BA1301-07. AUTHOR INFORMATION Author notes * These authors contributed equally: Sae-Geun Park, Yong-Cheol Jeong and
Dae-Gun Kim. AUTHORS AND AFFILIATIONS * Department of Biological Sciences, KAIST, Daejeon, Korea Sae-Geun Park, Yong-Cheol Jeong, Dae-Gun Kim, Min-Hyung Lee, Anna Shin, Geunhong Park, Jia
Ryoo, Jiso Hong, Seohui Bae & Daesoo Kim * Department of Mechanical Engineering, KAIST, Daejeon, Korea Dae-Gun Kim, Cheol-Hu Kim & Phill-Seung Lee Authors * Sae-Geun Park View author
publications You can also search for this author inPubMed Google Scholar * Yong-Cheol Jeong View author publications You can also search for this author inPubMed Google Scholar * Dae-Gun
Kim View author publications You can also search for this author inPubMed Google Scholar * Min-Hyung Lee View author publications You can also search for this author inPubMed Google Scholar
* Anna Shin View author publications You can also search for this author inPubMed Google Scholar * Geunhong Park View author publications You can also search for this author inPubMed Google
Scholar * Jia Ryoo View author publications You can also search for this author inPubMed Google Scholar * Jiso Hong View author publications You can also search for this author inPubMed
Google Scholar * Seohui Bae View author publications You can also search for this author inPubMed Google Scholar * Cheol-Hu Kim View author publications You can also search for this author
inPubMed Google Scholar * Phill-Seung Lee View author publications You can also search for this author inPubMed Google Scholar * Daesoo Kim View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS D.K. and P.-S.L. designed the study and coordinated the experiments. S.-G.P., Y.-C.J., G.P. and S.B. performed the behavioral experiments
with optogenetics. D.-G.K., C.-H.K., P-S.L. and Y.-C.J. developed the head-mounted device, MIDAS algorithm and navigation platform. Y.-C.J. and A.S. performed the electrophysiological
experiments. M.-H.L., J.R. and J.H. performed the histological experiments and analysis. All authors participated in writing the manuscript. CORRESPONDING AUTHORS Correspondence to
Phill-Seung Lee or Daesoo Kim. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. INTEGRATED SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 ELECTRICAL LESIONS OF THE ANTERIOR
HYPOTHALAMUS ABOLISH OBJECT-HOARDING BEHAVIOR IN LONG EVANS RATS. a. Schematic illustrating electrical lesioning of the rat anterior hypothalamus. Electrical lesion areas (gray) in five
representative brain sections are depicted. Scale bar, 1 mm. b. Schematic depiction of the hoarding behavior experimental protocol. c. Latency to hoard in sham and lesion groups. The lesion
group showed a significant object hoarding delay (Sham, _n_ = 8 rats, Lesion, _n_ = 7 rats; Mann Whitney U test, _P_ = 0.021). d. Total play-like object engagement was significantly reduced
in the lesion group (Sham, _n_ = 8 rats, Lesion, _n_ = 7 rats; Unpaired t-test, _P_ = 0.49). Bar graphs indicate mean (bars) ± SEM (error bars) and individual data (circles). See the
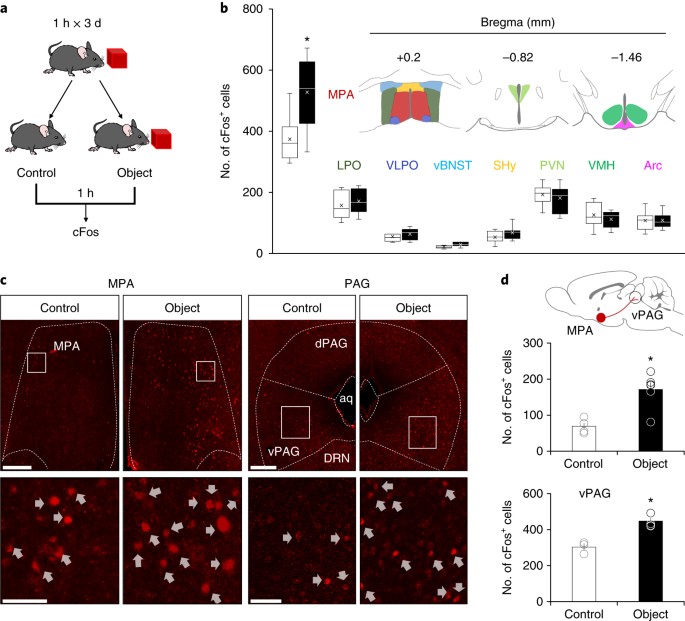
detailed statistical values in Supplementary table 1. * _P_ < 0.05 SUPPLEMENTARY FIGURE 2 IMMUNOHISTOCHEMICAL ANALYSIS OF CFOS ACTIVITY IN HYPOTHALAMIC NUCLEI UPON OBJECT EXPOSURE. A.
Representative immunohistochemistry (IHC, stained with DAB) images of cFos expression in the MPA. Scale bars, 200 µm. B. Magnified images (control, _n_ = 8 mice; object; _n_ = 7 mice). Scale
bars, 50 µm. C. Representative images from other hypothalamic regions (control, _n_ = 8 mice; object, _n_ = 7 mice). Scale bars, 100 µm. Abbreviations: LPO, lateral preoptic area; VLPO,
ventrolateral preoptic nucleus; vBNST, ventral bed nucleus of the stria terminalis; SHy, septohypothalamic nucleus; PVN, paraventricular nucleus; VMH, ventromedial hypothalamus; Arc, arcuate
nucleus; aca, anterior commissure (anterior part). See Figure 1b for the quantitative data SUPPLEMENTARY FIGURE 3 VALIDATION OF VIRALLY MEDIATED EXPRESSION OF CHR2 IN VGLUT2::CHR2MPA-VPAG
AND VGAT::CHR2MPA-VPAG MICE. A. _Top_, Schematic depiction of VGLUT2+ MPA-vPAG circuit photostimulation of _VGLUT2_-ires-_Cre_ mice. Bottom, Representative images of virally mediated ChR2
expression in the MPA and its termini in vPAG. 5 mice were used for these images. B. _Top_, Schematic illustration of photostimulation in _VGAT_-ires-_Cre_ mice. _Bottom_, Representative
images of ChR2 expression in MPA and vPAG of _VGAT_-ires-_Cre_ mice. 5 mice were used for these images. Scale bars, 200 µm SUPPLEMENTARY FIGURE 4 APPROPRIATE EXPRESSION OF CHR2 IN THE MPA
AND THE EFFECTIVE LOCATION OF FIBER OPTIC FIBER IN THE PAG. A. Representative image of virally mediated ChR2 expression in the MPA and surrounding brain regions. B. Representative image of
an individual mouse brain showing non-specific viral expression leading to reduced MPA-specific expression. Note that this mouse showed no response to objects during photostimulation. C.
Quantification of viral expression in the MPA and surrounding brain regions (_n_ = 17 mice; RM ANOVA on Ranks, MPA vs. LPO, _P_ < 0.05; MPA vs. VLPO, _P_ < 0.05; MPA vs. vBNST, _P_
< 0.05; MPA vs. SHy, _P_ < 0.05). D. Schematic depiction of retrograde tracing from vPAG. Bottom, Representative image of the MPA and surrounding brain regions. Note that labeled
neurons project to the vPAG. E. Representative image of retrograde tracing. 3 mice were used for this image. F. Quantification of labeled neurons in the MPA and surrounding brain regions
(_n_ = 3 mice; One Way RM ANOVA, MPA vs. LPO, _P_ = 0.002; MPA vs. VLPO, _P_ = 0.001; MPA vs. vBNST, _P_ = 0.001; MPA vs. SHy, _P_ = 0.002). G. Locations of the fiber optic cannulas in the
vPAG. The red crosses indicate the optic fiber tip locations in mice that responded to an object (_n_ = 6) during photostimulation of the MPA-vPAG circuit; the black crosses indicate tip
locations in mice that showed no response (_n_ = 3). Scale bars, 200 µm. Bar graphs indicate mean (bars) ± SEM (error bars) and individual data (circles). See the detailed statistical values
in Supplementary table 1. * _P_ < 0.05 SUPPLEMENTARY FIGURE 5 PHOTOSTIMULATION OF SOMATA AND AXON TERMINI OF CAMKIIΑ+ MPA NEURONS. A. Photostimulation of MPA neuron somata increases
object dislocation by CaMKIIα::ChR2MPA mice (_n_ = 6) more than control eYFPMPA mice (_n_ = 7; Mann Whitney U test, Light on state between two groups, _P_ = 0.008; Wilcoxon Signed Rank test,
ON vs. OFF in ChR2, _P_ = 0.031). B. Female CaMKIIα::ChR2MPA-vPAG mice (red lines; _n_ = 4) moved presented objects more during photostimulation than control CaMKIIα::eYFPMPA-vPAG mice
(gray lines; _n_ = 5; Two Way RM ANOVA, ON state between two groups, _P_ < 0.001; OFF vs. ON in ChR2, _P_ < 0.001; ON vs. OFF in ChR2, _P_ < 0.001). C. Bar graphs comparing object
dislocation during photostimulation of CaMKIIα MPA somata in male mice and of the CaMKIIα MPA-vPAG circuit in male and female mice (_n_ = 7 mice for MPA somata, _n_ = 5 for
CaMKIIα::ChR2MAP-vPAG male mice, _n_ = 4 for CaMKIIα::ChR2MAP-vPAG female mice; One Way ANOVA, MPA somata vs. CaMKIIα::ChR2MAP-vPAG male, _P_ < 0.001; CaMKIIα::ChR2MAP-vPAG male vs.
CaMKIIα::ChR2MAP-vPAG female, _P_ = 0.004). Line graphs represent mean (thick lines) ± SEM (error bars) and individual data (brighter lines). Bar graphs indicate mean (bars) ± SEM (error
bars) and individual data (circles). See the detailed statistical values in Supplementary table 1. * _P_ < 0.05 SUPPLEMENTARY FIGURE 6 ATTENTION SHIFT TO OBJECTS FROM FEMALES IN THE
CAMKIIΑ::CHR2MPA-VPAG MICE. A. Photostimulation elevates the locomotion of CaMKIIα::ChR2MPA-vPAG (_n_ = 8) mice but not in CaMKIIα::eYFPMPA-vPAG (_n_ = 5) mice (Unpaired t-test, _P_ =
0.008). B. Representative behavioral raster plots for the inter-male aggression test. Each behavior is color-coded as indicated. 6 mice were used for this image. C. Percentage of social
exploration and attacks versus non-social exploration behaviors (i.e., searching and digging) during encounters with an intruder male. Note that photostimulation reduced attacks toward the
intruder male and increased non-social exploration. Photostimulation did not alter social exploration behavior (_n_ = 6 for CaMKIIα::ChR2MPA-vPAG mice, Paired t-test, social exploration, _P_
= 0.308; attack, _P_ = 0.003; non-social exploration, _P_ = 0.007). Colored-boxes represent percentage of each behaviors. d. Duration of attacks by CaMKIIα::eYFPMPA-vPAG (_n_ = 6) and
CaMKIIα::ChR2MPA-vPAG (_n_ = 6) mice (Mann Whitney U test, _P_ = 0.026). E. Number of attacks by CaMKIIα::eYFPMPA-vPAG (_n_ = 6) and CaMKIIα::ChR2MPA-vPAG (_n_ = 6) mice (Unpaired t-test,
_P_ = 0.037). F. Percentage of time male CaMKIIα::ChR2MPA-vPAG (_n_ = 10) and CaMKIIα::eYFPMPA-vPAG (_n_ = 8) mice (Unpaired t-test, _P_ = 0.00000415) spent searching for females during
photostimulation. Note that, like what we observed in the inter-male aggression tests, CaMKIIα::ChR2MPA-vPAG mice spent less time searching because they spent more time in non-social
exploration. g. CaMKIIα::ChR2MPA-vPAG (_n_ = 6) eat less than CaMKIIα::eYFPMPA-vPAG (_n_ = 8) mice during photostimulation (Unpaired t-test, _P_ = 0.001). Bar graphs show mean (bars) ± SEM
(error bars) and individual data (circles). See the detailed statistical values in Supplementary table 1. * _P_ < 0.05 SUPPLEMENTARY FIGURE 7. _IN VIVO_ ACTIVITY OF THE CAMKIIΑ+ MPA–VPAG
CIRCUIT MEASURED BY PHOTOMETRY. A. The expression of GCAMP6m that mediated by viral infection of AAV9-CAG-DIO-GCaMP6m and AAV5-CaMKIIα::-Cre in MPA. 6 mice were used for these images. Scale
bars, 200 µm. B. Representative raw trace of fluorescent activity [\(\Delta F/F=(F-{F}_{mean})/{F}_{mean}\)] during object exploration. C. Success rates, which indicate increase of
fluorescent activity during object exploration, are not different across the bouts. (_n_ = 6 mice; One Way RM ANOVA, _P_ = 0.800) Bar indicate average of population and colored-lines
indicate individuals. D. Peak fluorescent activity of individuals runs down across the bout number. (_n_ = 6 mice) Bar graphs indicate mean (bars) ± SEM (error bars) and individual data
(colored-lines). Dot graphs represent mean (dots) ± SEM (error bars), trend line (green line) and individual data (circles). See the detailed statistical values in Supplementary table 1. *
_P_ < 0.05 SUPPLEMENTARY FIGURE 8. PHOTOINHIBITION OF THE CAMKIIΑ+ MPA–VPAG CIRCUIT ABOLISHES OBJECT EXPLORATION. A. _Left_, CaMKIIα::eNpHR3.0MPA-vPAG mice (_n_ = 8) move presented
objects less than control CaMKIIα::eYFPMPA-vPAG mice (_n_ = 5, Two Way RM ANOVA, 5 min, _P_ < 0.01; 10 min _P_ = 0.135; 15 min, _P_ = 0.643; 20 min, _P_ = 0.562). Line graph indicates
mean (lines) ± SEM (error bars). _Right_, Curves for individual mice. b. Total food intake. CaMKIIα::eYFPMPA-vPAG (_n_ = 5) and CaMKIIα::eNpHR3.0MPA-vPAG (_n_ = 6) mice (Unpaired t-test, _P_
= 0.936) eat similar amounts. Bar graphs represent mean (bars) ± SEM (error bars) and individual data (colored-lines). See the detailed statistical values in Supplementary table 1. * _P_
< 0.05, _n_.s. not significant SUPPLEMENTARY FIGURE 9 THE HARDWARE CONFIGURATION OF THE MIDAS. A. Photographs of the head-mounted device components and Bluetooth module. Scale bar, 1 cm.
B. Schematic for the overall system. The PC navigation program analyzes positional information for each mouse and for the 3D maze using a CMOS camera. It then transmits control commands to
the head-mounted device via Bluetooth. The controller chipset in the head-mounted device controls the target object servomotor and the LED module. C. The average mouse velocity in response
to photostimulation using different LED-ON angles (_n_ = 5 mice; One Way RM ANOVA, 30° vs. 10°, _P_ = 0.023; 30° vs. 40°, _P_ = 0.039; 30° vs. 70°, _P_ = 0.013). D. The average velocity from
the start to the goal as a function of frequency (_n_ = 3 mice; One Way RM ANOVA, 0 Hz vs. 40 Hz, _P_ < 0.001; 10 Hz vs. 40 Hz, _P_ < 0.001; 15 Hz vs. 40 Hz, _P_ < 0.001; 20 Hz vs.
40 Hz, _P_ = 0.008). Line graphs represent mean (thick lines) ± SEM (error bars) and individual data (brighter lines). See the detailed statistical values in Supplementary table 1. * _P_
< 0.05 SUPPLEMENTARY FIGURE 10 A COMPLEX MAZE FOR EVALUATING THE CONTROL OF MOUSE BEHAVIOR BY MIDAS. A. The maze consists of seven hurdles: ① a zig-zag course; ② a female distractor; ③
blind alleys; ④ rough terrain; ⑤ a mesh ladder; ⑥ food distractors; and ⑦ a bridge. B. Latency to the goal during a 300-s navigation session. Note that the OC+ LC condition showed
significantly less latency than the other conditions (_n_ = 5 mice; One Way RM ANOVA, OC only vs. OC+LC, _P_ < 0.001; LC only vs. OC+LC, _P_ < 0.001). C. Frequency of getting trapped
in the hurdles, quantified as described in the Methods. Note that mice in the OC+LC condition were trapped in the hurdles significantly less often than mice of the other conditions (_n_ = 5
mice; One Way RM ANOVA, OC only vs. OC+LC, _P_ = 0.009; LC only vs. OC+LC, _P_ = 0.008). Bar graphs indicate mean (bars) ± SEM (error bars) and individual data (circles). See the detailed
statistical values in Supplementary table 1. * _P_ < 0.05 SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–10 LIFE SCIENCES REPORTING SUMMARY SUPPLEMENTARY
TABLE 1 VIDEOS SUPPLEMENTARY VIDEO 1: Time-dependent responses of mice toward objects. A light cube was provided to a B6 mouse. Before habituation, the mouse showed awareness of the light
cube. With time, the mouse began to show active engagement, such as touching, biting and, in the late phase, dislocation. SUPPLEMENTARY VIDEO 2: Photostimulation of the CaMKIIα+ MPA–vPAG
circuit induces strong object explorative behaviors. Photostimulation of the MPA–vPAG circuit (CaMKIIα::ChR2MPA-vPAG) induced a stronger object exploration compared with that observed in
CaMKIIα::ChR2MPA and CaMKIIα::eYFPMPA-vPAG control mice. SUPPLEMENTARY VIDEO 3: Photostimulation of CaMKIIα+ MPA neurons induces object explorative behaviors. Photostimulation of the MPA led
to increased engagement with the presented object in a mouse transfected with ChR2 in the MPA (CaMKIIα::ChR2MPA) but not one transfected with control virus (CaMKIIα::eYFPMPA). Behavioral
changes were evident between the light-OFF and light-ON states. SUPPLEMENTARY VIDEO 4: In vivo calcium recording of the CaMKIIα+ MPA–vPAG circuit during object exploration. _In vivo_ calcium
recording was conducted in the vPAG to record signals from the axon termini of CaMKIIα+ MPA neurons expressing GCaMP6m. Note. The black bar located on the left side of the movie indicates
ΔF/F in real-time. It turns red when the ΔF/F exceeds 3σ of the session. SUPPLEMENTARY VIDEO 5: Photostimulation of male CaMKIIα::ChR2MPA-vPAG mice shifts their attention from a female to a
target object. During the light-OFF state, a male CaMKIIα::ChR2MPA-vPAG mouse (C57BL/6J) spent more time searching for a female (BALB/cJ) than for a presented object. In the light-ON state,
the male began to show more active engagement with the object rather than the female. SUPPLEMENTARY VIDEO 6: Exploratory behaviors for various 3D objects in CaMKIIα::ChR2MPA-vPAG mice.
Photostimulation of the CaMKIIα+ MPA–vPAG circuit led to object-specific manipulation that depended on the size and shape of the object. Objects examined included a ping-pong ball, cotton
bud, wooden block and rubber cap. Video shows behavior during the light-ON state. SUPPLEMENTARY VIDEO 7: Photostimulation of the CaMKIIα+ MPA–vPAG circuit triggers object-craving behaviors.
Photostimulation of the MPA–vPAG circuit induces object-craving behaviors under various conditions. First, a CaMKIIα::ChR2MPA-vPAG mouse leaps down from a precipice to grasp the object.
Second, a CaMKIIα::ChR2MPA-vPAG mouse jumps to grasp the object in the air, reaching out with its paws and mouth. Even when the object was presented above water, the mouse tried to catch it
by moving around the edge of the water. SUPPLEMENTARY VIDEO 8: Object-chasing behavior of CaMKIIα::ChR2MPA-vPAG mice toward a moving object. CaMKIIα::ChR2MPA-vPAG mice exposed to an object
moving along the contours of the letters B and G (representing our Behavioral Genetics laboratory) followed the object during the light-ON state. When the light was turned off right before
the mouse reached the letter G, the mouse stopped following the object and moved away from the G track. SUPPLEMENTARY VIDEO 9: Photostimulation of the CaMKIIα+ MPA–vPAG circuit induces
hunting-like actions to prey. CaMKIIα::ChR2MPA-vPAG mice exposed to a prey (cricket) under photostimulation. They showed series of hunting-like behaviors, such as chasing, biting and
carrying. SUPPLEMENTARY VIDEO 10: Steering of mouse navigation in a complex maze using the MIDAS system. The video shows a schematic overview of the complex maze and a CaMKIIα::ChR2MPA-vPAG
mouse running the maze. _Left_, Top view of the maze (yellow circle, mouse; pink circle, object; black circle, waypoints; blue circle, current waypoint; red line, programmed pathway to the
goal; blue line, current guiding direction; black line, actual track of the mouse). _Top right_, Close-up video of the maze. The names of the hurdles are shown. _Bottom right_, Actual mouse
tracks and object positions, updated in real time (red circle, current object position). Note that the LED is turned on only when the object is positioned in the center of the five circles.
RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Park, SG., Jeong, YC., Kim, DG. _et al._ Medial preoptic circuit induces hunting-like actions to target
objects and prey. _Nat Neurosci_ 21, 364–372 (2018). https://doi.org/10.1038/s41593-018-0072-x Download citation * Received: 18 April 2017 * Accepted: 09 December 2017 * Published: 29
January 2018 * Issue Date: March 2018 * DOI: https://doi.org/10.1038/s41593-018-0072-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative