Autophagy activated by silibinin contributes to glioma cell death via induction of oxidative stress-mediated bnip3-dependent nuclear translocation of aif
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Induction of lethal autophagy has become a strategy to eliminate glioma cells, but it remains elusive whether autophagy contributes to cell death via causing mitochondria damage and
nuclear translocation of apoptosis inducing factor (AIF). In this study, we find that silibinin induces AIF translocation from mitochondria to nuclei in glioma cells in vitro and in vivo,
which is accompanied with autophagy activation. In vitro studies reveal that blocking autophagy with 3MA, bafilomycin A1 or by knocking down ATG5 with SiRNA inhibits silibinin-induced
mitochondrial accumulation of superoxide, AIF translocation from mitochondria to nuclei and glioma cell death. Mechanistically, silibinin activates autophagy through depleting ATP by
suppressing glycolysis. Then, autophagy improves intracellular H2O2 via promoting p53-mediated depletion of GSH and cysteine and downregulation of xCT. The increased H2O2 promotes
silibinin-induced BNIP3 upregulation and translocation to mitochondria. Knockdown of BNIP3 with SiRNA inhibits silibinin-induced mitochondrial depolarization, accumulation of mitochondrial
superoxide, and AIF translocation from mitochondria to nuclei, as well as prevents glioma cell death. Furthermore, we find that the improved H2O2 reinforces silibinin-induced glycolysis
dysfunction. Collectively, autophagy contributes to silibinin-induced glioma cell death via promotion of oxidative stress-mediated BNIP3-dependent nuclear translocation of AIF. SIMILAR
CONTENT BEING VIEWED BY OTHERS INHIBITION OF MTOR SIGNALING PROTECTS HUMAN GLIOMA CELLS FROM HYPOXIA-INDUCED CELL DEATH IN AN AUTOPHAGY-INDEPENDENT MANNER Article Open access 06 October 2022
TARGETING SIRT3 SENSITIZES GLIOBLASTOMA TO FERROPTOSIS BY PROMOTING MITOPHAGY AND INHIBITING SLC7A11 Article Open access 23 February 2024 ACTIVATED SIRT1 CONTRIBUTES TO DPT-INDUCED GLIOMA
CELL PARTHANATOS BY UPREGULATION OF NOX2 AND NAT10 Article 05 June 2023 INTRODUCTION Glioma is an aggressive malignant brain tumor and constitutes the major causes leading to death in both
pediatric and adult populations1. The median survival time of the patients with newly diagnosed glioma is only 14.6 months, despite these patients are treated with surgical removal of tumor
followed by radiotherapy and chemotherapy with temozolomide (TMZ)2. As an alkylating agent, TMZ is widely used for treating primary and recurrent high-grade gliomas, but its efficacy is
often limited by development of resistance3. Therefore, new medicines are needed for glioma treatment. Autophagy that is characterized morphologically with formation of autophagosomes or
autolysosomes in cytoplasm is a degradation pathway by which intracellular materials or impaired organelles are delivered to lysosomes for clearance4. Autophagy plays dual roles in
regulation of cell destiny. On the one hand, it protects cells against detrimental stresses. On the other hand, it contributes to cell death, which is designated as autophagic death4. It was
found that autophagy protected neuroblastoma cells by removing misfolded proteins, which were generated during the process of oxidative stress5. Autophagy was also reported to abrogate
cisplatin-induced death in hepatocellular carcinoma cells via inhibiting accumulation of reactive oxygen species (ROS) by clearing damaged mitochondria6. Thus, the protection of autophagy
against cell damage is closely associated with suppression of oxidative stress. In the case of autophagic death, autophagy is also accompanied with accumulation of intracellular ROS and
generally thought to be activated by ROS7,8. However, it remains elusive whether autophagy could promote cell death via improving intracellular ROS. Mitophagy refers to selective removal of
damaged mitochondria via autophagy machinery9. Although mitophagy rescues cell death via clearing injured mitochondria6, accumulating evidence showed that excessive or sustained mitophagy
could also lead to cell death10,11. It was reported that ceramide triggered glioma cell death via overactivation of mitophagy10. Moreover, mitophagy was found to contribute to the glioma
cell death induced by chemical compound AT10111. Given that only damaged mitochondria could be recognized and degraded via autophagy pathway9, it remains elusive why excessive clearance of
damaged mitochondria by autopahgy results in cell death. This makes us speculate that overactivated autophagy might play a role in causing mitochondrial damage. Silibinin is a biologically
active component of silymarin that is a polyphenolic extract from milk thistle (Silybum marianum) (Fig. 1a). It has been widely used to treat and prevent many types of hepatobiliary
disorders including alcoholic liver disease, nonalcoholic fatty liver disease, and mushroom poisoning12,13. Moreover, silibinin not only exert potent inhibitory effect on various types of
cancer cells, such as breast cancer cells, prostate cancer cells, and glioma cells7,14,15, but also sensitizes glioma cells to TMZ, TRAIL or arsenic trioxide treatment and prevents
hepatocyte injury induced by chemotherapy agent cisplatin16,17,18,19. Although silibinin could induce autophagic death in cancer cells7,8, the biochemical events downstream silibinin-induced
autophagy remains unclear. Therefore, in this study, we used glioma cell lines and nude mice with xenografted glioma to investigate whether autophagy plays a role in promoting mitochondria
damage and its potential mechanism. MATERIALS AND METHODS REAGENTS Silibinin was purchased from MedChemExpress (Monmouth Junction, NJ). Glutathione (GSH) was from Sigma (St.Louis, MO).
Silibinin was dissolved in DMSO to a storage concentration of 200 mmol/L. GSK-872 was from Calbiochem company (Billerica, MA, USA). GKT137831, necrostatin-1(Nec-1), NSA (Necrosulfonamide),
and ferrostatin-1(Fer-1) were from selleckchem Company (Houston, TX). Deferoxamine was from Abcam company (Cambridge, MA). Anti-AIF (#4642) and phospho-p53(Ser15) (#9284) antibody were from
cell signaling company (Danvers, MA). The primary antibodies against the following proteins ATG5(ab109490), LC3B(192890) p62/SQSTM1(ab56416), xCT(ab175186), BNIP3(ab10433), PKM2(ab150377),
HK II(ab209847), PFKP(ab119796), HIF-1α (ab51608), GPX4(ab125066), p53(ab26), TOMM20(ab186735), H2A(ab177308), and β-Actin (ab8226) were all purchased from Abcam company (Cambridge, MA).
Other reagents were purchased from Sigma (St. Louis, MO). CELL LINE AND CULTURE Human U87, U251, SHG-44, and rat C6 glioma cells were all obtained from Shanghai Institute of Cell Biology,
Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, penicillin (100 U/mL) and streptomycin (100
μg/mL), and maintained at 37 °C and 5% CO2 in a humid environment. Cells in the mid-log phase were used in the experiments. CELLULAR VIABILITY AND CELL DEATH ASSAYS U87 (5 × 104 cells/well),
U251 (5 × 104 cells/well), SHG-44 (5 × 104 cells/well), and C6 (1 × 105 cells/well) glioma cells were treated with target compounds after being seeded onto 96-well microplate with six
duplicated wells and cultured 24 h. Cellular viability was assessed using an MTT assay, and was expressed as a ratio to the absorbance value at 570 nm of the control cells. Cell death was
assessed by using lactate dehydrogenase cytotoxicity assay kit according to the manufacturer’s instructions (Beyotime Biotech, Nanjing, China), the absorbance value of each sample was read
at 490 nm, and cell death ratio was calculated by using the following formula: cell death ratio % = (A sample − A control/A max − A control) × 100. A sample: sample absorbance value; A
control: the absorbance value of control group; A max: the absorbance value of positive group. MEASUREMENT OF MITOCHONDRIAL MEMBRANE POTENTIAL AND MITOCHONDRIAL SUPEROXIDE Mitochondrial
membrane potential was assayed by using JC-1 staining (Beyotime Biotech, Nanjing, China) as described by manufacture’s instruction. The collected cells were analyzed by flow cytometry
(FACScan, Becton Dickinson, San Jose, CA). Mitochondrial superoxide was assayed by using MitoSOX red according to manufacturer’s description (Invitrogen, Eugene, OR). The red fluorescence
density was measured at an excitation wavelength of 510 nm and an emission wavelength at 580 nm, and was expressed as a ratio to the fluorescence in control cells. The cells seeded on a
six-well plate were treated as the same as above described, and then observed under fluorescence microscope (Olympus IX71, Tokyo, Japan). All the measurements and observation were performed
by a researcher who was blinded to group allocation and repeated for five times. MEASUREMENT OF INTRACELLULAR H2O2 CONTENT The content of intracellular H2O2 in treated glioma cells was
analyzed with a H2O2 assay kit (Beyotime Institute of Biotechnology, Nanjing, China) according to the manufacturer protocol. In brief, the cells were collected by centrifugation at 800 × _g_
for 5 min and washed twice with PBS. The cell pellets and 10 mg xenograft glioma tissues were lysed in lysis buffer by repeated cycles of freezing and thawing under liquid nitrogen and
centrifuged at 12,000 × _g_ for 5 min. Then, 50 mL of supernatants and 100 mL of test solution were added into a tube, placed at room temperature for 30 min, and measured immediately with a
spectrophotometer at a wavelength of 560 nm. Absorbance values were calibrated to a standard concentration curve to calculate the concentration of H2O2. The measurement was performed by a
researcher who was blinded to group allocation and repeated for five times. Finally, the results were expressed as a ratio to the concentration of the control cells. TRANSFECTION OF SMALL
INTERFERING RNA (SIRNA) The cells were seeded onto a culture dish. Transfection of siRNA was performed by using Lipofectamine 2000 (Invitrogen, Eugene, OR) according to the manufacturer’s
instructions. ATG5 SiRNA (5′-GACGUUGGUAACUGACAAATT-3′), BNIP3 SiRNA (5′- GAUUACUUCUGAGCUUGCATT-3′), AIF SiRNA (5′-GCAGUGGCAAGUUACUUAUTT-3′) and scrambled SiRNA (5′-UUCUCCGAACGUGUCACGUTT-3′)
were all purchased from GenePharma Company (Suzhou, China). After SiRNA transfection overnight, the cells were incubated with silibinin at indicated dosage for subsequent experiments. RAT C6
TUMOR XENOGRAFT IN MICE The female athymic BALB/c nude mice (age 4 weeks, weight 20–22 g, Beijing Vital River laboratory animal technology company, China) were housed in a specific
pathogen-free environment under the condition of 12-h light/12-h dark cycle, free access to food and water, and acclimatized to their surroundings for 3 days. The mice were cared in
accordance with the guidelines for experimental animals of Jilin University and the study was approved by the ethics committee of First Hospital of Jilin University (Changchun, China). The
animal quantity used in this study was estimated as described previously20. A total of 1 × 107 logarithmically growing C6 cells in 100 μL of PBS were subcutaneously injected into the right
flank of each mouse. After 7 days, the mice with similar tumor size (about 150 mm3) were randomly allocated to control group (_n_ = 5) and treatment group (_n_ = 5). The treatment group
received intraperitoneal injections of silibinin at the dosage of 100 mg/kg body weight each day for 12 days, and the control group received vehicle in the same volume. The tumor size was
measured by a researcher who was blinded to the group allocation with a slide caliper, and the tumor volume was calculated using the formula: 0.5 × A × B2, in which A is the length of the
tumor and B is the width. On the next day of the last treatment, the mice were euthanized by cervical dislocation. After being excised and weighed, the tumors were frozen immediately in
liquid nitrogen for western blotting analysis. DIFFERENTIAL CENTRIFUGATION, GEL ELECTROPHORESIS, AND WESTERN BLOTTING The collected glioma cells by centrifugation and the frozen xenografted
glioma tissue were homogenized with a glass Pyrex microhomogenizer (20 strokes) in ice cold lysis buffer (Beyotime Biotech, Nanjing, China). Homogenates were centrifuged at 1000 × _g_ for 10
min at 4 °C to obtain the supernatant 1 and the pellet 2. The supernatant 1 was then centrifuged at 10,000 × _g_ for 10 min at 4 °C to obtain supernatant 2 and pellet 2. The pellet 1 was
nuclear fraction, supernatant 1 was cytoplasmic fraction, pellet 2 was mitochondrial fraction, and supernatant 2 was cytoplasmic fraction without mitochondria. The protein content was
determined using Bio-Rad protein assay kit. After SDS electrophoresis and transfer to PVDF membranes, the membranes were blocked with 3% BSA in TBS for 30 min at room temperature, and then
incubated overnight at 4 °C with primary antibodies. After incubation with horseradish peroxidase-conjugated secondary antibody and washing the blots, immunoreactive proteins were visualized
on a chemi-luminescence developer (ChemiScope 5300, Clinx Scicence Instrument Company, Shanghai) and then the density was quantified by using software of Image J. The procedure was
performed by a researcher who was blinded to group allocation. IMMUNOCYTOCHEMICAL STAINING The cells seeded on a culture dish were fixed in ethanol, washed with PBS, and incubated with
1%Triton X-100 for 10 min. The cells were incubated with 100 nmol/L Mitotracker red ((Invitrogen company, Eugene, OR)) for 30 min at 37 °C before fixation in ethanol. After the nonspecific
antibody binding sites were blocked, the cells were incubated with anti-BNIP3 (1:100) or anti-AIF antibody (1:100) followed by incubation in Alexa Fluor 488-conjugated goat anti-rabbit IgG
(1:200) for 1 h and then with Heochst33258. Finally, all the cells were visualized under laser scanning confocal microscope (Olympus FV1000, Tokyo, Japan) by a researcher who was blinded to
group allocation. MEASUREMENT OF GSH, CYSTEINE, ATP, GLUCOSE-6-PHOPHATE, AND PYRUVATE Intracellular total GSH was measured by using a DTNB-GSSH reductase recycling assay kit (Beyotime
Biotechnology, Nanjing, China) as described by manufacture. Briefly, the collected cells and frozen glioma tissue were resuspended in protein-removing buffer S and lysed by repeated cycles
of freezing and thawing under liquid nitrogen. The cell lysates were centrifuged at 10,000 × _g_ for 10 min at 4 °C to get the supernatant used for assay. GSH content was expressed as a
ratio to the absorbance value at 412 nm of the control cells. Intracellular cysteine was measured by using a cysteine assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China)
according to the manufacturer protocol. Briefly, the collected cells were added into reagent A, homogenized on ice, and centrifuged at 8000 × _g_ for 4 min at 4 °C to obtain the supernatant
for assay. After the protein concentration was measured, 20 μL sample was incubated with 100 μL reagent B and 100 μL reagent C for 15 min at room temperature and read at absorbance 600 nm in
a microplate reader. Finally, the results were expressed as a ratio to the absorbance value of the control cells. Glucose-6-phosphate, pyruvate, and ATP measurement were all performed
according to manufacturer instructions of Biovision company (Milpitas, CA). Briefly, the cells were collected by centrifugation or 10 mg xenograft glioma tissues were lysed in assay buffer
by repeated sonification to be prepared to samples for examinations. For glucose-6-phosphate assay, the samples were mixed with respective reaction buffers and readings were taken at 450 nm.
For measurement of the concentration of pyruvate and ATP, the samples were mixed with respective reaction buffers and read at absorbance 570 nm in a microplate reader. All the measurement
was performed by researchers who were blinded to group allocation and repeated for five times. Finally, the results were expressed as a ratio to the absorbance value of the control cells.
STUBRFP-SENSGFP-LC3 ASSAY The StubRFP-SensGFP-LC3 assay was performed according to manufacturer’s instruction (Genechem, Shanghai China). Briefly, the U87 cells (5 × 104 cells/mL) seeded on
a culture dish were incubated with the StubRFP-SensGFP-LC3B lentivirus (MOI = 5 × 106 TU/mL). New medium was changed after 12 h of incubation. At transinfection 72 h, the cells were treated
with silibinin, and then incubated with lysotracker blue or mitotracker deep red (Invitrogen company, Eugene, OR). Finally, the cells were observed by a researcher who was blinded to group
allocation with a confocal laser scanning microscope (Olympus, Tokyo, Japan). STATISTICAL ANALYSIS All data represent at least four independent experiments and are expressed as mean ± SD.
Statistical comparisons were made using one-way ANOVA. _P_ values of less than 0.05 were considered to represent statistical significance. RESULTS AUTOPHAGY CONTRIBUTED TO SILIBININ-INDUCED
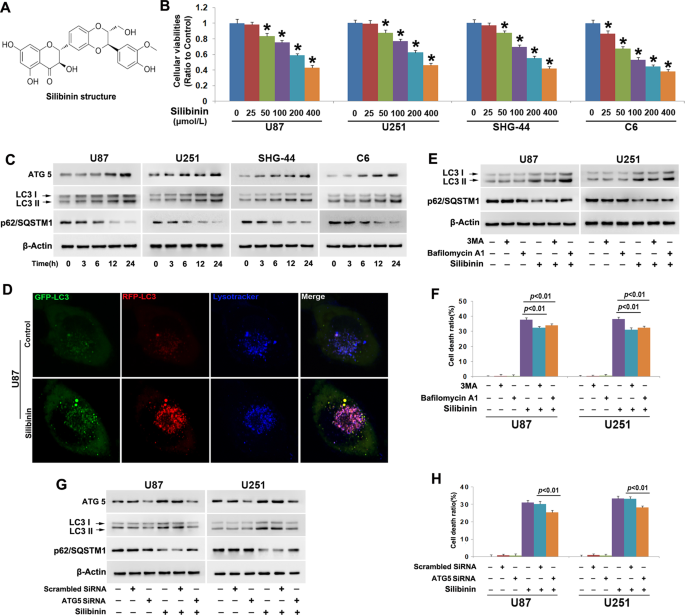
GLIOMA CELL DEATH To evaluate the toxic effect of silibinin on glioma cells, we used MTT assay to assess cellular viability. As shown in Fig. 1b, treatment with silibinin at indicated
concentrations for 24 h resulted in obvious decreases in the cellular viabilities of U87, U251 and SHG-44 and C6 glioma cells, and the reduction of cellular viability became more apparent
with the increase of silibinin concentration. This indicated that silibinin inhibited glioma cell viability in a dosage-dependent manner. Then, we calculated the IC50 values of silibinin at
24 h and found that they were 272.1 μmol/L in U87 cells, 261.6 μmol/L in U251 cells, 246.1 μmol/L in SHG-44 cells, and 162.5 μmol/L in C6 cells. Thus, 200 μmol/L was used in the subsequent
studies. To address the mechanism accounting for the inhibitory effect of silibinin on glioma cells, we investigated whether silibinin induced activation of lethal autophagy. As revealed by
western blotting analysis, silibinin triggered time-dependent increases of autophagy marker proteins ATG5 and LC3-II, and corresponding reduction of autophagy substrate p62 (SQSTM1) (Fig. 1c
and Fig. S1A–C). Then, the U87 cells transinfected with stubRFP-sensGFP-LC3 lentiviruses were used to evaluate whether autophagy flux was activated by silibinin in glioma cells. As revealed
by confocal microscopy combined with lysosome probe lysotracker blue staining, many red pucta and green punta were found to form in the cytoplasm of the cells treated with silibinin at 200
μmol/L for 10 h, when compared with control cells (Fig. 1d). Although some red puncta were colocalized with green puncta to display yellow color, the other red puncta were colocalized with
blue puncta to present violet color (Fig. 1d). Because green fluorescence is prone to be quenched in the acid environment of lysosome lumen, the yellow puncta were autophagosomes and the
violet puncta were autolysosomes. Therefore, these results indicated that silibinin activated autophagy. To assay the role of autophagy in silibinin-induced glioma cell death, U87 and U251
cells were treated for 1 h with 3MA that inhibits autophagy at initiation stage or bafilomycin A1 that disturbs autophagosome to fuse with lysosome, and then incubated with silibinin at 200
μmol/L for 24 h. Western blotting analysis showed that silibinin-induced upregulation of LC3-II and downregulation of p62 (SQSTM1) were both obviously inhibited by pretreatment with 5 mmol/L
3MA. Despite the reduction of p62 (SQSTM1) induced by silibinin was markedly suppressed in the cells pretreated with 1.5 μmol/L bafilomycin A1, the protein level of LC3-II was further
improved (Fig. 1e, Fig. S1D and E). Then, LDH release assay demonstrated that silibinin-induced death in glioma cells was significantly abrogated in the presence of 3MA or bafilomycin A1
(Fig. 1f). To further verify the role of autophagy in silibinin-induced glioma cell death, we knocked down ATG5 with SiRNA and examined its effect on glioma cell death. It was found that
knockdown of ATG5 not only prevented silibinin-induced upregulation of LC3-II and downregulation of p62 (SQSTM1) (Fig. 1g, Fig. S1F–H), but also inhibited glioma cell death (Fig. 1h).
Therefore, these results indicated that silibinin induced autophagic death in glioma cells. Furthermore, we used SHG-44 cells to investigate whether silibinin induced necroptosis and
ferroptosis in glioma cells. As shown by LDH assay, 1 h pretreatment with necroptosis specific inhibitor Nec-1 (100 μmol/L), GSK-872 (20 μmol/L), and NSA (10 μmol/L) did not prevent
silibinin-induced glioma cell death (Fig. S1I). Additionally, pretreatment with ferroptosis inhibitor deferoxamine (500 μmol/L) and Fer-1 (20 μmol/L) did not inhibit the glioma cell death
induced by silibinin as well (Fig. S1J). Thus, these results indicated that neither necroptosis nor ferroptosis was induced in glioma cells by silibinin. AUTOPHAGY CONTRIBUTED TO
SILIBININ-INDUCED MITOCHONDRIA DAMAGE To address whether silibinin induces damage in mitochondrion, we measured mitochondrial membrane potential by using JC-1 and mitochondrial superoxide by
using Mitosox red. JC-1is a probe emitting red fluorescence when accumulates in healthy mitochondria but green fluorescence when is released from damaged mitochondria into cytoplasm. As
revealed by fluorescence microscopy, the red fluorescence exhibited by JC-1 decreased drastically in the cells treated with silibinin at 200 μmol/L for 12 h, when compared with control cells
(Fig. 2a). This was also demonstrated by the results acquired by flow cytometry analysis combined with JC-1 staining, which showed that silibinin caused time-dependent reduction in red
fluorescence (Fig. 2b, Fig. S2A). These indicated that silibinin induced mitochondria depolarization in a time-dependent manner. Then, we found that the red fluorescence exhibited by Mitosox
red which is a specific probe for mitochondrial superoxide was much brighter in the cells treated with silibinin at 200 μmol/L for 24 h than that in control cells (Fig. 2c). Statistical
analysis demonstrated as well that the red fluorescence intensity was improved with the extension of silibinin treatment (Fig. 2d). This indicated that silibinin induced time-dependent
accumulation of mitochondrial superoxide. Therefore, these data suggested that silibinin treatment resulted in mitochondrial damage. Considering that AIF translocation from depolarized
mitochondria to nuclei could lead to cell death, we thus isolated mitochondrial and nuclear fractions and examined silibinin-induced changes in distribution of AIF. As revealed by western
blotting, when compared with control cells, AIF was decreased in mitochondria fractions, whereas increased in nuclear fractions by silibinin in a time-dependent manner (Fig. 2e, Fig. S2B–D).
Confocal microscopy showed as well that AIF accumulated more apparently in the nuclei of the cells incubated with silibinin for 24 h than that in control ones (Fig. 2f). To clarify the role
of AIF in silibinin-induced glioma cell death, we introduce SiRNA to knock down AIF and examined its effect on glioma cell death by using LDH release assay. It was found that
silibinin-induced upregulation of AIF in nuclear fractions was mitigated as a consequence of the overall reduction of AIF expression in the cells transfected with AIF SiRNA when compared
with the cells transfected with scrambled SiRNA (Fig. 2g, Fig. S2E–G). Moreover, silibinin-induced glioma cell death was also suppressed significantly when AIF was knocked down with SiRNA
(Fig. 2h). Therefore, these indicated that silibinin triggered AIF-dependent death in glioma cells. Furthermore, we found that inhibition of autophagy with 3MA or bafilomycin A1 obviously
abrogated silibinin-induced accumulation of mitochondrial superoxide and AIF translocation from mitochondria to nuclei (Fig. 2i and j, Fig. S2H–J). Similar results could be found when ATG5
was knocked down with SiRNA (Fig. 2k and l, Fig. S2K–M). Therefore, these results suggested that autophagy contributed to silibinin-induced mitochondria damage and nuclear translocation of
AIF. AUTOPHAGY REINFORCED SILIBININ-INDUCED BNIP3 ACCUMULATION ON MITOCHONDRIA Given that BNIP3 (Bcl-2/adenovirus E1B 19-kDa-interacting protein 3) is protein targeting mitochondria and
could induce mitochondrial damage and nuclear translocation of AIF6, we thus isolated mitochondrial fractions from U87, U251, SHG-44, and C6 cells and examined silibinin-induced changes in
BNIP3. As shown by western blotting, silibinin not only induced BNIP3 overexpression, but also improved BNIP3 levels in mitochondrial fractions in a time-dependent manner (Fig. 3a, Fig. S3A
and B). Confocal microscopy also confirmed that BNIP3 accumulated more obviously on mitochondria in the cells treated with silibinin for 24 h than that in control cells (Fig. 3b). These
indicated that silibinin promoted BNIP3 accumulation on mitochondria, as well as upregulated its expression in glioma cells. To elucidate the role of BNIP3 in silibinin-produced toxicity in
glioma cells, we used SiRNA to knock down BNIP3 and examined its effect on glioma cell death and mitochondrial damage. As revealed by western blotting, silibinin-induced BNIP3 overexpression
and its accumulation on mitochondria were both inhibited in the cells transfected with BNIP3 SiRNA, when compared with the cells transfected with scrambled SiRNA (Fig. 3c, Fig. S3C and D).
Moreover, LDH release assay proved that knockdown of BNIP3 with SiRNA significantly prevented the glioma cell death induced by silibinin (Fig. 3d). Furthermore, silibinin-induced
accumulation of mitochondrial superoxide, mitochondria depolarization, and AIF translocation from mitochondria to nuclei were all inhibited when BNIP3 was knocked down with SiRNA (Fig. 3e–g,
Fig. S3E–G). Thus, these indicated that BNIP3 contributed to silibinin-induced glioma cell death via causing mitochondrial damage and nuclear translocation of AIF. Notably, we found that
silibinin-induced overexpression of BNIP3 and BNIP3 upregulation in mitochondrial fractions were both inhibited in the cells pretreated with 3MA or bafilomycin A1 (Fig. 3h, Fig. S3H and I).
This indicated that autophagy promoted silibinin-induced BNIP3 overexpression and its accumulation on mitochondria. This might be a factor accounting for the promotion effect of autophagy on
silibinin-induced mitochondria. AUTOPHAGY AUGMENTED SILIBININ-INDUCED ACCUMULATION OF H2O2 To address the mechanism accounting for the regulatory effect of silibinin on BNIP3, we examined
silibinin-induced changes in HIF-1α and hydrogen peroxide given that HIF-1α is a transcription factor of BNIP3 and BNIP3 could also be upregulated under the condition of oxidative stress6.
Thus, we isolated nuclear fractions by using differential centrifugation and examined the protein level of HIF-1α in the absence and presence of silibinin by western blotting. When compared
that in control cells, nuclear level of HIF-1α was downregulated by silibinin in a time-dependent manner (Fig. 4a, Fig. S4A). However, intracellular hydrogen peroxide was markedly improved
time-dependently by silibinin, which was accompanied with GSH depletion at each indicated time point (Fig. 4b). Considering that NADPH oxidase (Nox) plays a role in promoting the generation
of hydrogen peroxide, the cells were pretreated with Nox inhibitor GKT137831 at 500 μmol/L for 1 h and then incubated with silibinin for 24 h. It was found that GKT13831 not only inhibited
silibinin-induced improvement of hydrogen peroxide, but also prevented glioma cell death (Fig. S4B and C). This indicated that Nox might be involved in regulation silibinin-induced
overproduction of hydrogen peroxide. Furthermore, we found that silibinin treatment also resulted in time-dependent upregulation of glutathione peroxidase 4 (GPX4) (Fig. 4a, Fig. S4D). Given
that GSH could be used by GPX4 to clear hydrogen peroxide17, the glioma cells were supplemented with exogenous GSH to verify the role of hydrogen peroxide in silibinin-induced changes in
BNIP3. It was found that pretreatment with GSH at 10 mmol/L for 1 h obviously inhibited the improvement of hydrogen peroxide induced by silibinin at indicated dosages (Fig. 4d). Moreover,
LDH release assay demonstrated as well that silibinin-induced glioma cell death was abrogated significantly by supplement of GSH (Fig. 4e). Then, it was revealed by western blotting that
silibinin-induced overexpression of BNIP3 in cytoplasmic and mitochondrial fractions were both inhibited in the presence of GSH (Fig. 4f, Fig. S4E and F). To further confirm the role of
hydrogen peroxide in promoting BNIP3 expression and accumulation on mitochondria, we treated U87 and U251 cells with exogenous hydrogen peroxide at 300 μmol/L for indicated time and analyzed
the distribution of BNIP3 by western blotting. It was found that hydrogen peroxide not only upregulated BNIP3 expression, but also improved BNIP3 levels in mitochondrial fractions in a
time-dependent manner (Fig. 4g, Fig. S4G and F). These results indicated that GSH depletion contributed to silibinin-induced improvement of hydrogen peroxide, which resulted in BNIP3
upregulation and accumulation on mitochondria. Additionally, we found that pretreatment with 3MA or bafilomycin A1 not only reversed silibinin-induced GSH depletion, but also prevented
accumulation of hydrogen peroxide (Fig. 4h and i). Therefore, these results suggested that autophagy contributed to silibinin-induced changes in BNIP3 via causing GSH depletion-dependent
accumulation of hydrogen peroxide. AUTOPHAGY PROMOTED SILIBININ-INDUCED PHOSPHORYLATION OF P53 To elucidate why silibinin treatment leads to GSH depletion, we examined silibinin-induced
changes in cysteine because it is a material used for GSH synthesis21. As shown in Fig. 5a, intracellular cysteine was decreased by silibinin in a time-dependent manner, when compared with
that in control cells. Considering that cysteine is transformed from cystine whose transportation into cells depends on cystine/glutamate antiporter21, we used western blotting to analyze
silibinin-induced changes in the protein level of xCT (SLC7A11) and phosphorylation of p53. xCT is a specific light-chain subunit of cystine/glutamate antiporter and its expression is
negatively regulated by phosphorylated p5321. We found that silibinin downregulated xCT, but upregulated p53 and phospho-p53 in a time-dependent manner (Fig. 5b, Fig. S5A–C). These indicated
that silibinin activated p53, which resulted in downregulation of xCT and depletion of cysteine. In contrast, the downregulation of xCT and the phosphorylation of p53 induced by silibinin
were both inhibited apparently when the cells were pretreated with 3MA or bafilomycin A1 (Fig. 5c, Fig. S5D–F). Moreover, silibinin-induced depletion of cysteine was correspondingly
prevented in the presence of 3MA or bafilomycin A1 (Fig. 5d). Similar results could be found when ATG5 was knocked down with SiRNA (Fig. 5e and f). Thus, these data indicated that autophagy
contributed to silibinin-induced depletion of cysteine via promotion of p53 activation. SILIBININ SUPPRESSED GLYCOLYSIS IN GLIOMA CELLS To elucidate the mechanism accounting for
silibinin-induced autophagy activation, we investigated whether silibinin induces glycolysis dysfunction considering that glycolysis is the primary energy-generating pathway in cancer cells
and energy failure could lead to autophagy activation4. It was found that silibinin triggered time-dependent depletion of ATP (Fig. 6a). Moreover, glycose-6-phosphate, which is the first
product of glycolysis generated by hexokinase II (HK II) and pyruvate that is the final product of glycolysis produced by pyruvate kinase 2 (PKM2) were both depleted by silibinin in a
time-dependent manner (Fig. 6b and c). These indicated that silibinin suppressed glycolysis in glioma cells. Then, western blotting was used to analyze silibinin-induced changes in HK II,
6-phosphofructokinase (PFKP), and PKM2, which are known to regulate the three rate-limiting steps of glycolysis. We found that the protein levels of HK II, PFKP, and PKM2 were all
downregulated time-dependently by silibinin in U87, U251, SHG-44, and C6 glioma cells (Fig. 6d, Fig. S6A–C). This indicated that silibinin suppressed glycolysis via downregulation of HK II,
PFKP, and PKM2. To test the role of hydrogen peroxide in silibinin-induced glycolysis dysfunction, U87 and U251 cells were treated with antioxidant GSH at 10 mmol/L for 1 h and then
incubated with silibinin at 200 μmol/L for 24 h. It was found that silibinin-induced reduction of ATP, glucose-6-phosphate, and pyruvate were all inhibited by supplement of GSH (Fig. 6e–g).
Western blotting analysis revealed that pretreatment with GSH obviously abrogated the downregulation of HK II, PFKP, and PKM2 induced by silibinin at indicated dosages (Fig. 6h, Fig. S6D–F).
Correspondingly, silibinin-induced increases of autophagy marker proteins ATG5 and LC3-II and decrease of autophagy substrate p62 (SQSTM1) were all inhibited in the presence of GSH (Fig.
6h, Fig. S6G–I). Thus, these data not only indicated that hydrogen peroxide reinforced the inhibitory effect of silibinin on glycolysis, but also indirectly suggested that glycolysis
dysfunction led to silibinin-induced autophagy activation. SILIBININ INHIBITED GLIOMA CELL GROWTH IN VIVO To verify the treatment effect of silibinin on glioma cells in vivo, C6 cells were
xenografted subcutaneously into the flank of nude mice. After being treated with silibinin at the dosage of 100 mg/kg each day for consecutive 12 days, the volumes of the xenografted tumors
were found to decrease markedly when compared with those in control group (Fig. 7a). This was also confirmed by statistical analysis of tumor volumes (Fig. 7b). Then, the tumors removed from
the sacrificed mice were homogenized and silibinin-induced changes in autophagy marker proteins were analyzed by western blotting. When compared with the control group, silibinin triggered
obviously upregulation of ATG5 and LC3-II, but reduction of p62 (SQSTM1) (Fig. 7c, Fig. S7A). This indicated that silibinin induced autophagy activation in glioma cells in vivo. Moreover, it
was found that hydrogen peroxide was at higher level, whereas GSH and cysteine were both at lower levels in silibinin-treated group than control group (Fig. 7d–f). Consistently, western
blotting analysis revealed that xCT was markedly downregulated, but p53 and phospho-p53 were both obviously upregulated by silibinin (Fig. 7g, Fig. S7B). This indicated silibinin-induced
accumulation of hydrogen peroxide, depletion of GSH and cysteine, downregulation of xCT and activation of p53 in glioma cells in vivo. Furthermore, we found that silibinin not only
upregulated the expression of BNIP3, but also improved BNIP3 levels in mitochondrial fractions (Fig. 7h, Fig. S7C). Although AIF was decreased by silibinin in mitochondrial fractions, it was
increased apparently in nuclear fractions (Fig. 7i, Fig. S7D). This indicated that BNIP3 might play a role in regulation of silibinin-induced nuclear translocation of AIF in glioma cells in
vivo. Additionally, it was found that silibinin treatment resulted in depletion of ATP, glucose-6-phosphate, and pyruvate (Fig. 7j–l) and downregulation of HK II, PFKP, and PKM2 (Fig. 7m,
Fig. S7E). This indicated that silibinin triggered energy failure via suppression of glycolysis in glioma cells in vivo. DISCUSSION In summary, we found in this study that silibinin induced
AIF translocation from mitochondria to nuclei in glioma cells in vitro and in vivo, which was accompanied with autophagy activation. In vitro studies revealed that blocking autophagy with
3MA1, bafilomycin A1 or by knocking down ATG5 with SiRNA inhibited silibinin-induced mitochondrial accumulation of superoxide, AIF translocation from mitochondria to nuclei and glioma cell
death. Mechanistically, silibinin activated autophagy through depleting ATP by suppressing glycolysis. Then, autophagy improved H2O2 via promoting p53-mediated depletion of GSH and cysteine
and downregulation of xCT. The increased H2O2 not only contributed to silibinin-induced BNIP3 upregulation and translocation to mitochondria, but also exacerbated glycolysis dysfunction.
Knockdown of BNIP3 with SiRNA inhibited silibinin-induced mitochondrial depolarization, accumulation of mitochondrial superoxide, and AIF translocation from mitochondria to nuclei, as well
as prevented glioma cell death. Collectively, autophagy contributed to silibinin-induced glioma cell death via causing BNIP3-mediated mitochondria damage and nuclear translocation of AIF
(Fig. 8). Overactivation of autophagy has been established to be an effective way to eradicate cancer cells7,8,22, and silibinin is confirmed to be a potent lethal autophagy inducer for
various types of cancer cells such as human breast cancer cells, fibrosarcoma cells, and renal carcinoma cells7,8,23. Different from previous report showing that silibinin triggered
protective autophagy in glioma cells24, we found in this study that blocking autophagy with 3MA, bafilomycin A1 or by knocking down ATG5 with SiRNA significantly rescued silibinin-induced
glioma cell death. This verified that autophagy contributed to silibinin-induced death in glioma cells. Moreover, different types of programmed cell death could be induced by the same
chemical or under the same pathological condition. Previous reports have shown that silibinin treatment could also trigger apoptosis in glioma cells15. Additionally, accumulating evidences
have demonstrated that autophagic death could occur simultaneously with other types of programmed cell death, such as ferroptosis and necroptosis25,26. Thus, induction of autophagic death is
a pathway accounting for the toxic effect of silibinin on glioma cells. Energy failure is a key factor initiating autophagy via activation of AMPK/mTOR pathway4. Glycolysis regulated by
three rate-limiting enzymes HK II, PFKP, and PKM2 is usually used by cancer cells to acquire ATP20. Suppression of glycolysis by pharmacological inhibition of HK II and genetic knockdown of
PKM2 were respectively reported to causing cancer cell death via excessive activation of autophagy27,28. In this study, we found that treatment with silibinin not only obviously reduced ATP
level, but also depleted glucose-6-phosphate and pyruvate and downregulated the protein levels of HK II, PFKP, and PKM2. This suggested that silibinin induced energy failure in glioma cells
via suppressing glycolysis, which was also supported by the study showing that silibinin suppressed glycolytic activity in pancreatic cancer cells29. Although we did not further investigate
whether AMPK/mTOR pathway was activated by silibinin, AMPK/mTOR activation was reported to account for silibinin-triggered autophagic death in renal carcinoma cells23. Thus, we thought that
silibinin induced lethal autophagy in glioma cells via suppression of glycolysis. Despite it was generally accepted that the protection of autophagy against cell death is associated with
inhibition of oxidative stress by clearing damaged mitochondria5, several studies have shown that autophagy plays a role in promotion of oxidative stress. Autophagy was reported to improve
intracellular ROS levels via selective degradation of catalase, which is an enzyme responsible for clearing hydrogen peroxide30. Moreover, it was also found that HK II which accounts for
generating glucose-6-phosphate during the process of glycolysis is also a selective substrate of autophagy31. Notably, knockdown of HK II with SiRNA was confirmed to cause accumulation of
intracellular ROS32. Thus, these studies revealed that autophagy also plays an important role in improving intracellular ROS levels. Accumulating evidences showed as well that oxidative
stress was responsible for silibinin-induced death in various types of cancer cells8,12,33. Recent studies proved that activation of p53 is a primary pathway via which silibinin improved
intracellular ROS levels. Activated p53 promoted silibinin-induced oxidative stress mainly through two pathways, one is triggering excessive generation of ROS in mitochondria by activation
of JNK, and the other is causing depletion of antioxidant GSH34,35. Of note, activated p53 was reported to deplete GSH via negative regulation of cysteine level and xCT expression21. In this
study, we found that blocking autophagy with 3MA or bafilomycin A1 not only inhibited silibinin-induced phosphorylation of p53, but also prevented silibinin-triggered downregulation of xCT,
depletion of cysteine and GSH and improvement of hydrogen peroxide. Although we did not investigate in this study the role of autophagy in silibinin-induced activation of p53, it was
reported that autophagy promoted p53 activation via selectively degradation of Δ133p53α (an inhibitory p53 isoform that can inhibit full-length p53)36. Therefore, our data suggested that
autophagy contributed to silibinin-induced oxidative stress in glioma cells via promotion of p53 activation. Additionally, it was also reported that glycolysis was inhibited significantly
when glioma cells were treated with hydrogen peroxide20. In this study, we found that the inhibitory effect of silibinin on glycolysis was apparently reversed when hydrogen peroxide was
suppressed by supplement of GSH. Therefore, silibinin also induced a positive feedback between glycolysis dysfunction and autophagy, which resulted in excessive activation of autophagy.
Autophagy was reported to account for the glioma cell death induced by ceremide or compound AT101 via excessively removing mitochondria10,11, whereas accumulating evidences have shown that
only damaged mitochondria, which are separated from mitochondrial network could be recognized, encapsulated and then degraded via autophagy pathway37. Thus, mitochondria damage is prior to
its removal by autophagy. It has been demonstrated that apoptosis, necroptosis, and parthanatos contributed to cell death via causing mitochondrial damage and nuclear translocation of
AIF38,39,40, but it remains elusive whether autophagy plays a role in impairing mitochondria and promoting AIF translocation from mitochondria to nuclei. In this study, we found that
blocking autophagy with 3MA, bafilomycin A1 or by knocking down ATG5 with SiRNA not only inhibited silibinin-induced glioma cell death, but also prevented mitochondrial accumulation of
superoxide and nuclear translocation of AIF. As a protein located within the space between mitochondria outer membrane and inner membrane, AIF is only released from damaged mitochondria,
which had depleted membrane potential41,42. Within nucleus, AIF acts as a nuclease after cooperating with cyclophilin A to form a DNA-degrading complex at the location of DNA double strand
breaks, which eventually leads to irreversible chromatinolysis and cell death41,42. Consistent with previous report showing that silibinin induced glioma cell death via promotion of AIF
translocation from mitochondria to nuclei15, our data in this study proved that knockdown of AIF with SiRNA not only prevented silibinin-induced its accumulation in nuclei, but also
inhibited glioma cell death. Thus, autophagy contributes to silibinin-induced glioma cell death via causing mitochondria damage and AIF translocation form mitochondria to nuclei. Previously,
intracellular ROS were found to play a crucial role in promotion of mitochondria damage and AIF translocation from mitochondria to nuclei. It was reported that treatment with hydrogen
peroxide obviously triggered mitochondrial depolarization and nuclear translocation of AIF in glioma cells40. Therefore, these studies suggested that improvement of intracellular hydrogen
peroxide is a pathway via which autophagy promoted silibinin-induced mitochondrial damage. As a protein targeting mitochondria, BNIP3 is excessively expressed in human gliomas43. BNIP3 plays
dual roles in regulation of cell demise. Suppression of BNIP3 by upregulation of miR-145 was reported to induce apoptosis in glioma cells43. However, BNIP3 upregulation could also lead to
cell death. It was reported that overexpressed BNIP3 contributed to baicalein-induced apoptosis in osteosarcoma cells, hydrogen peroxide-induced autophagic death in glioma cells, and
doxorubicin-induced necrosis in cardiac myocytes44,45. As well as Bax, Bim and Noxa which induce mitochondrial dysfunction upon accumulation on mitochondria46,47,48, BNIP3 was confirmed to
trigger MPTP opening and loss of mitochondrial membrane potentials when its C-terminal transmembrane domain is inserted into mitochondrial outer membrane10. Jiang et al. proved that BNIP3
was involved in regulation of silibinin-induced reduction of mitochondrial membrane potentials in human breast cancer cells7. Consistently, we found in this study that silibinin not only
upregulated the protein level of BNIP3, but also promoted BNIP3 translocate to mitochondria. In contrast, knockdown of BNIP3 with SiRNA obviously inhibited silibinin-induced mitochondrial
depolarization, accumulation of mitochondrial superoxide and nuclear translocation of AIF, as well as rescued glioma cell death. Thus, BNIP3 contributed to silibinin-induced glioma cell
death via causing mitochondrial damage. Although HIF-1α is a transcription factor regulating BNIP3 expression49, we found in this study that silibinin treatment resulted in time-dependent
downregulation of HIF-1α. This was also supported by previous study showing silibinin inhibited HIF-1α expression in prostate cancer cells14. Oxidative stress was also reported to contribute
to baicalein-induced BNIP3 upregulation in human osteosarcoma cells44. Consistently, we found as well that silibinin-induced BNIP3 upregulation and translocation to mitochondria were both
apparently reversed when hydrogen peroxide was suppressed by supplement of GSH or autophagy was blocked. Given that silibinin-induced improvement of hydrogen peroxide was inhibited in the
presence of 3MA or bafilomycin A1, we think that autophagy promoted silibinin-induced damage in mitochondria via regulation of BNIP3. In conclusion, we demonstrated in this study that
silibinin activates lethal autophagy though suppression of glycolysis. Autophagy improves intracellular hydrogen peroxide via depletion of GSH and cysteine by promoting p53 phosphorylation.
Ultimately, hydrogen peroxide triggers BNIP3-dependnet mitochondria damages and nuclear translocation of AIF. Therefore, autophagy contributes to silibinin-induced glioma cell death via
causing mitochondrial damage and nuclear translocation of AIF. DATA AVAILABILITY The data supporting the findings of this study are available within the article and supplementary files or
from the authors upon reasonable request. REFERENCES * Lapointe, S., Perry, A. & Butowski, N. A. Primary brain tumours in adults. _Lancet_ 392, 432–446 (2018). Article PubMed Google
Scholar * Messaoudi, K., Clavreul, A. & Lagarce, F. Toward an effective strategy in glioblastoma treatment. Part I: resistance mechanisms and strategies to overcome resistance of
glioblastoma to temozolomide. _Drug Discov. Today_ 20, 899–905 (2015). CAS PubMed Google Scholar * Yan, Y. et al. Targeting autophagy to sensitive glioma to temozolomide treatment. _J.
Exp. Clin. Cancer Res._ 35, 23 (2016). PubMed PubMed Central Google Scholar * Levy, J., Towers, C. G. & Thorburn, A. Targeting autophagy in cancer. _Nat. Rev. Cancer_ 17, 528–542
(2017). CAS PubMed Google Scholar * Awan, M. U. et al. Neuroprotective role of BNIP3 under oxidative stress through autophagy in neuroblastoma cells. _Mol. Biol. Rep._ 41, 5729–5734
(2014). CAS PubMed Google Scholar * Su, Y. C. et al. Galectin-1-induced autophagy facilitates cisplatin resistance of hepatocellular carcinoma. _PLoS ONE_ 11, e0148408 (2016). PubMed
PubMed Central Google Scholar * Jiang, K. et al. Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7
breast cancer cells. _Oncol. Rep._ 33, 2711–2718 (2015). CAS PubMed PubMed Central Google Scholar * Duan, W. J. et al. Silibinin activated ROS-p38-NF-κB positive feedback and induced
autophagic death in human fibrosarcoma HT1080 cells. _J. Asian Nat. Prod. Res._ 13, 27–35 (2011). CAS PubMed Google Scholar * Ney, P. A. Mitochondrial autophagy: origins, significance,
and role of BNIP3 and NIX. _Biochim. Biophys. Acta_ 1853, 2775–2783 (2015). CAS PubMed Google Scholar * Dany, M. & Ogretmen, B. Ceramide induced mitophagy and tumor suppression.
_Biochim. Biophys. Acta_ 1853, 2834–2845 (2015). CAS PubMed PubMed Central Google Scholar * Meyer, N. et al. AT 101 induces early mitochondrial dysfunction and HMOX1 (heme oxygenase 1)
to trigger mitophagic cell death in glioma cells. _Autophagy_ 14, 1693–1709 (2018). CAS PubMed PubMed Central Google Scholar * Hosseinabadi, T. et al. Silymarin antiproliferative and
apoptotic effects: insights into its clinical impact in various types of cancer. _Phytother. Res._ 33, 2849–2861 (2019). CAS PubMed Google Scholar * Abenavoli, L. et al. Milk thistle
(Silybum marianum): a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. _Phytother. Res._ 32, 2202–2213 (2018). PubMed Google Scholar * Jung, H.
J. et al. Silibinin inhibits expression of HIF-1alpha through suppression of protein translation in prostate cancer cells. _Biochem. Biophys. Res. Commun._ 390, 71–76 (2009). CAS PubMed
Google Scholar * Jeong, J. C. et al. Silibinin induces apoptosis via calpain-dependent AIF nuclear translocation in U87MG human glioma cell death. _J. Exp. Clin. Cancer Res._ 30, 44 (2011).
CAS PubMed PubMed Central Google Scholar * Elhag, R., Mazzio, E. A. & Soliman, K. F. The effect of silibinin in enhancing toxicity of temozolomide and etoposide in p53 and
PTEN-mutated resistant glioma cell lines. _Anticancer Res_. 35, 1263–1269 (2015). * Son, Y. G. et al. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5
up-regulation and down-regulation of c-FLIP and survivin. _Cancer Res._ 67, 8274–8284 (2007). CAS PubMed Google Scholar * Gülden, M. et al. Chrysin and silibinin sensitize human
glioblastoma cells for arsenic trioxide. _Food Chem. Toxicol._ 105, 486–497 (2017). PubMed Google Scholar * Yang, Z. et al. Silibinin restores the sensitivity of cisplatin and taxol in
A2780-resistant cell and reduces drug-induced hepatotoxicity. _Cancer Manag Res._ 11, 7111–7122 (2019). CAS PubMed PubMed Central Google Scholar * Lu, B. et al. RIP1 and RIP3 contribute
to shikonin-induced glycolysis suppression in glioma cells via increase of intracellular hydrogen peroxide. _Cancer Lett._ 425, 31–42 (2018). CAS PubMed Google Scholar * Imai, H.,
Matsuoka, M., Kumagai, T., Sakamoto, T. & Koumura, T. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. _Curr. Top. Microbiol. Immunol._ 403, 143–170 (2017). CAS
PubMed Google Scholar * Fu, J., Shao, C. J., Chen, F. R., Ng, H. K. & Chen, Z. P. Autophagy induced by valproic acid is associated with oxidative stress in glioma cell lines.
_Neuro-Oncol._ 12, 328–340 (2010). CAS PubMed Google Scholar * Li, F. et al. Autophagy induction by silibinin positively contributes to its anti-metastatic capacity via AMPK/mTOR pathway
in renal cell carcinoma. _Int. J. Mol. Sci._ 16, 8415–8429 (2015). CAS PubMed PubMed Central Google Scholar * Bai, Z. L., Tay, V., Guo, S. Z., Ren, J. & Shu, M. G. Silibinin induced
human glioblastoma cell apoptosis concomitant with autophagy through simultaneous inhibition of mTOR and YAP. _Biomed. Res. Int._ 2018, 6165192 (2018). PubMed PubMed Central Google Scholar
* Zhou, B. et al. Ferroptosis is a type of autophagy-dependent cell death. _Semin. Cancer Biol._ S1044-579X, 30006–30009 (2019). Google Scholar * Goodall, M. L. et al. The autophagy
machinery controls cell death switching between apoptosis and necroptosis. _Dev. Cell_ 37, 337–349 (2016). CAS PubMed PubMed Central Google Scholar * Zhang, Q. et al. Hexokinase II
inhibitor, 3-BrPA induced autophagy by stimulating ROS formation in human breast cancer cells. _Genes Cancer_ 5, 100–112 (2014). PubMed PubMed Central Google Scholar * Dey, P. et al. PKM2
knockdown induces autophagic cell death via AKT/mTOR pathway in human prostate cancer cells. _Cell. Physiol. Biochem._ 52, 1535–1552 (2019). CAS PubMed Google Scholar * Shukla, S. K. et
al. Silibinin-mediated metabolic reprogramming attenuates pancreatic cancer-induced cachexia and tumor growth. _Oncotarget_ 6, 41146–41161 (2015). PubMed PubMed Central Google Scholar *
Yu, L. et al. Autophagic programmed cell death by selective catalase degradation. _Proc. Natl Acad. Sci. USA_ 103, 4952–4957 (2006). CAS PubMed PubMed Central Google Scholar * Jiao, L.
et al. Regulation of glycolytic metabolism by autophagy in liver cancer involves selective autophagic degradation of HK2 (hexokinase 2). _Autophagy_ 14, 671–684 (2018). CAS PubMed Google
Scholar * Wu, R. et al. Hexokinase II knockdown results in exaggerated cardiac hypertrophy via increased ROS production. _EMBO Mol. Med._ 4, 633–646 (2012). CAS PubMed PubMed Central
Google Scholar * Ham, J., Lim, W., Bazer, F. W. & Song, G. Silibinin stimluates apoptosis by inducing generation of ROS and ER stress in human choriocarcinoma cells. _J. Cell. Physiol._
233, 1638–1649 (2018). CAS PubMed Google Scholar * Fan, S. et al. P53 activation plays a crucial role in silibinin induced ROS generation via PUMA and JNK. _Free Radic. Res._ 46, 310–319
(2012). CAS PubMed Google Scholar * Fan, S. et al. P53-mediated GSH depletion enhanced the cytotoxicity of NO in silibinin-treated human cervical carcinoma HeLa cells. _Free Radic. Res._
46, 1082–1092 (2012). CAS PubMed Google Scholar * Horikawa, I. et al. Autophagic degradation of the inhibitory p53 isoform Δ133p53α as a regulatory mechanism for p53-mediated senescence.
_Nat. Commun._ 5, 4706 (2014). CAS PubMed Google Scholar * Ni, H. M., Williams, J. A. & Ding, W. X. Mitochondrial dynamics and mitochondrial quality control. _Redox Biol._ 4, 6–13
(2015). CAS PubMed Google Scholar * Fan, W. H. et al. Proteasome inhibitor MG-132 induces C6 glioma cell apoptosis via oxidative stress. _Acta Pharmacol. Sin._ 32, 619–625 (2011). CAS
PubMed PubMed Central Google Scholar * Ding, Y. et al. MLKL contributes to shikonin-induced glioma cell necroptosis via promotion of chromatinolysis. _Cancer Lett._ 467, 58–71 (2019). CAS
PubMed Google Scholar * Zheng, L. et al. JNK activation contributes to oxidative stress-induced parthanatos in glioma cells via increase of intracellular ROS production. _Mol.
Neurobiol._ 54, 3492–3505 (2017). CAS PubMed Google Scholar * Artus, C. et al. AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX.
_EMBO J._ 29, 1585–1599 (2010). CAS PubMed PubMed Central Google Scholar * Candé, C. et al. AIF and cyclophilin A cooperate in apoptosis-associated chromatinolysis. _Oncogene_ 23,
1514–1521 (2004). PubMed Google Scholar * Du, Y. et al. MicroRNA-145 induces apoptosis of glioma cells by targeting BNIP3 and Notch signaling. _Oncotarget_ 8, 61510–61527 (2017). PubMed
PubMed Central Google Scholar * Ye, F. et al. Baicalein induces human osteosarcoma cell line MG-63 apoptosis via ROS-induced BNIP3 expression. _Tumour Biol._ 36, 4731–4740 (2015). CAS
PubMed Google Scholar * Dhingra, R. et al. Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. _Proc. Natl Acad. Sci. USA_
111, E5537–5544 (2014). CAS PubMed PubMed Central Google Scholar * Shi, L. et al. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced
apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. _Brain Res._ 1352, 255–264 (2010). CAS PubMed Google Scholar * Stojcheva, N. et al. MicroRNA-138 promotes acquired
alkylator resistance in glioblastoma by targeting the Bcl-2-interacting mediator BIM. _Oncotarget_ 7, 12937–12950 (2016). PubMed PubMed Central Google Scholar * He, Y. et al. Regulation
of integrated stress response sensitizes U87MG glioblastoma cells to temozolomide through the mitochondrial apoptosis pathway. _Anat. Rec. (Hoboken)_ 301, 1390–1397 (2018). CAS Google
Scholar * Niecknig, H. et al. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. _Free Radic. Res._ 46, 705–717 (2012). CAS PubMed
Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by National Nature and Science Foundation of China (81372697, 81772669, and 81972346), Changbaishan Scholar
Project of Jilin province (2013026), Scientific Research Foundation of Jilin province (20150414013GH, 20200201405JC and 20190701051GH), and Foundation of the Health Department of Jilin
province (2018Q026). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neurosurgery, First Hospital of Jilin University, 130021, Changchun, China Chongcheng Wang, Chuan He, Shan
Lu, Xuanzhong Wang, Lei Wang, Shipeng Liang & Pengfei Ge * Research Center of Neuroscience, First Hospital of Jilin University, 130021, Changchun, China Chongcheng Wang, Chuan He, Shan
Lu, Xuanzhong Wang, Lei Wang, Shipeng Liang & Pengfei Ge * Department of Radiotherapy, Second Hospital of Jilin University, 130021, Changchun, China Xinyu Wang * Department of
Anesthesiology, First Hospital of Jilin University, 130021, Changchun, China Meihua Piao * Department of Histology and Embryology, College of Basic Medical Sciences, Jilin University,
130021, Changchun, China Jiayue Cui * Key Laboratory of Pathobiology, Ministry of Education, Jilin University, 130021, Changchun, China Guangfan Chi Authors * Chongcheng Wang View author
publications You can also search for this author inPubMed Google Scholar * Chuan He View author publications You can also search for this author inPubMed Google Scholar * Shan Lu View author
publications You can also search for this author inPubMed Google Scholar * Xuanzhong Wang View author publications You can also search for this author inPubMed Google Scholar * Lei Wang
View author publications You can also search for this author inPubMed Google Scholar * Shipeng Liang View author publications You can also search for this author inPubMed Google Scholar *
Xinyu Wang View author publications You can also search for this author inPubMed Google Scholar * Meihua Piao View author publications You can also search for this author inPubMed Google
Scholar * Jiayue Cui View author publications You can also search for this author inPubMed Google Scholar * Guangfan Chi View author publications You can also search for this author inPubMed
Google Scholar * Pengfei Ge View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS This study was conceived, designed, and interpreted by G.P.
and C.G. W.C., H.C., L.S., W.X., W.L., L.S., C.J., and P.M. undertook the data acquisition and analysis. C.G. and C.J. were responsible for the comprehensive technical support. G.P. and W.C.
were major contributors in writing the manuscript. C.J. and C.G. contributed to the inspection of data and final manuscript. All authors read and approved the final manuscript.
CORRESPONDING AUTHOR Correspondence to Pengfei Ge. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S
NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Edited by G.M. Fimia SUPPLEMENTARY INFORMATION LEGENDS FOR
SUPPLEMENTARY FIGURES FIGURE S1 FIGURE S2 FIGURE S3 FIGURE S4 FIGURE S5 FIGURE S6 FIGURE S7 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution
4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and
the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s
Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, C., He, C., Lu, S. _et al._ Autophagy activated by silibinin contributes to
glioma cell death via induction of oxidative stress-mediated BNIP3-dependent nuclear translocation of AIF. _Cell Death Dis_ 11, 630 (2020). https://doi.org/10.1038/s41419-020-02866-3
Download citation * Received: 13 May 2020 * Revised: 28 July 2020 * Accepted: 29 July 2020 * Published: 14 August 2020 * DOI: https://doi.org/10.1038/s41419-020-02866-3 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative